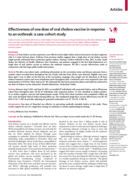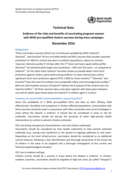Filter
4725
Filtered Results: 4725
Text search:
epidemiológica
Featured
Recommendations
411
New Publications
1071
Language
Document type
No document type
2281
Studies & Reports
871
Guidelines
821
Manuals
247
Strategic & Response Plan
242
Fact sheets
108
Situation Updates
73
Training Material
46
Resource Platforms
16
Infographics
10
Online Courses
7
Brochures
2
Dashboards/Maps
1
Countries / Regions
Global
220
Latin America and the Carribbean
175
India
164
Brazil
133
Western and Central Europe
119
Sierra Leone
90
Colombia
83
Liberia
80
Paraguay
74
Africa
73
Venezuela
69
South Africa
67
Kenya
66
Ethiopia
66
Nigeria
63
Argentina
62
Mozambique
55
Rwanda
49
Uganda
49
Eastern Europe
49
Nepal
47
Ghana
44
Bolivia
42
Peru
41
Russia
41
Congo, Democratic Republic of
39
Bangladesh
39
Tanzania
38
Ukraine
37
Zambia
37
Malawi
36
Guinea
36
Ecuador
31
Chile
29
Myanmar / Burma
29
Asia
28
West and Central Africa
27
South–East Asia Region
27
El Salvador
24
Syria
24
Namibia
24
East and Southern Africa
23
Senegal
22
Germany
22
Zimbabwe
20
Philippines
20
Haiti
20
Yemen
19
Angola
19
Middle East and North Africa
18
Eastern Europe and Central Asia
18
Indonesia
18
Madagascar
17
Pakistan
17
Cambodia
16
Lesotho
14
Benin
12
Cameroon
12
North America
12
Albania
11
Western Pacific Region
11
Spain
11
Somalia
11
Tajikistan
10
Burkina Faso
10
Mali
10
Botswana
10
China
9
Afghanistan
9
Guinea-Bissau
9
Côte d’Ivoire / Ivory Coast
8
Central African Republic
8
Honduras
8
South Sudan
8
Eswatini/ Swaziland
8
Sudan
7
Guatemala
7
Togo
6
Vietnam
6
Moldova
6
Georgia
6
Gambia
5
Niger
5
Dominican Republic
5
Jordan
5
Lebanon
5
Sri Lanka
5
Chad
5
Thailand
4
Mexico
4
Kyrgyzstan
4
Italy
4
Armenia
4
USA
4
Saudi Arabia
4
France
4
Portugal
4
Southern Africa
4
Iraq
4
North Macedonia
3
Timor Leste/ East Timor
3
Iran
3
Burundi
3
Djibouti
3
Egypt
3
Palestine
3
Serbia
2
Turkey
2
Kazakhstan
2
Laos
2
Estonia
2
Uzbekistan
2
Ireland
2
Uruguay
2
Turkmenistan
2
Jamaica
2
Mauritius
2
United Kingdom
2
Nicaragua
2
Greece
2
Poland
2
Libya
2
Bhutan
2
North Korea
1
Australia
1
Singapore
1
Congo-Brazzaville
1
Morocco
1
Qatar
1
Bulgaria
1
Mauritania
1
Lithuania
1
Maldives
1
Tunisia
1
Belarus
1
Belize
1
Costa Rica
1
Panama
1
Cuba
1
Authors & Publishers
Publication Years
Category
Countries
1757
Clinical Guidelines
387
Public Health
263
Key Resources
155
Women & Child Health
115
Pharmacy & Technologies
59
Capacity Building
41
Toolboxes
COVID-19
703
TB
426
HIV
365
NTDs
316
Rapid Response
297
Mental Health
273
Ebola & Marburg
180
Planetary Health
133
AMR
118
Zika
111
Malaria
98
NCDs
91
Polio
55
Cholera
53
Conflict
50
Disability
48
Global Health Education
47
Caregiver
44
Health Financing Toolbox
41
Pharmacy
40
Refugee
38
Natural Hazards
27
Specific Hazards
19
Social Ethics
5
Typhoon
2
Interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac
These WHO interim recommendations for use of the Sinovac-CoronaVac were developed on the basis of advice issued by the Strategic Advisory Group of Experts on Immunization (SAGE) and the evidence summary included in the background document and annexes referenced below.
This document has been updat...
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 117: e210277chgsa, 2022. online | memorias.ioc.fiocruz.br
Since May 2019, Confirmation of 4 new emergence of type 2 vaccine-derived poliovirus (VDPV2) in Bambari (2) and Bimbo (2) health districts without any genetic link between them and other known viruses ; Bambari Health District: CAF-RS4-BAM-19-058, onset of paralysis: May 02, 2019. 07 positive contac...
Anxiety disorders
Chapter F.3
The review’s objectives are to review progress in TB control with emphasis on DOTS strategy implementation, summarize the experience, lessons learnt and methods of work and to make recommendations for international donors, technical agencies and the Ministry of Health.
Olashore et al.
Child Adolesc Psychiatry Ment Health (2017) 11:8 DOI 10.1186/s13034-017-0144-9
While much progress has been achieved over the past year, the Region of the Americas has stubbornly remained the epicenter of the COVID-19 pandemic. PAHO is launching its 2021 COVID-19 Response Strategy and Donor Appeal to continue supporting Latin American and Caribbean countries and territories i...
Guidelines for the Management of Typhoid Fever
recommended
Saudi Journal of Biological Sciences. http://dx.doi.org/10.1016/j.sjbs.2016.03.006
Open Access
Cervical cancer is the fourth most common cancer in women worldwide in 2018, with 570,000 new cases and 311,000 deaths occurring annually.T he highest incidence rates are in Southern Africa, Eastern Africa, SubSaharan Africa, Western Africa, Melanesia, and Middle Africa . It also ranks as the leadin...
Lancet Glob Health 2016; 4: e856–63. Open Access
The article "Effectiveness of one dose of oral cholera vaccine in response to an outbreak: a case-cohort study" investigates whether a single dose of the Shanchol oral cholera vaccine can provide effective protection during an outbreak. Conducted i...
A tutorial for healthcare professionals
doi: https://doi.org/10.1101/2020.10.28.20221143
This article is a preprint and has not been peer-reviewed [what does this mean?]. It reports new medical research that has yet to be evaluated and so should not be used to guide clinical practice.
The technical note from the Global Task Force on Cholera Control (GTFCC) examines the risks and benefits of vaccinating pregnant women with WHO-prequalified oral cholera vaccines (OCVs) during mass vaccination campaigns. It highlights that three WHO-approved vaccines (Dukoral®, Shanchol™, and Euv...
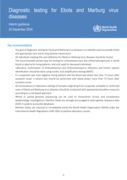
- The goal of diagnostic testing for Ebola and Marburg virus diseases is to identify cases to provide timely and appropriate care and to stop disease transmission.
- All individuals meeting the case definition for Ebola or Marburg virus diseases should be tested.
- The recommended sample type ...