Filter
32
Featured
3
Language
Document type
10
10
5
2
2
1
1
1
Countries / Regions
6
2
2
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
11
PLoS One 20(6): e0324338.https://doi.org/10.1371/journal.pone.0324338
AIDS Research and Therapy, vol.22 (2025) article no. 48. A 2025 research article reports on task shifting HIV disclosure support from clinical staff to caregiver peer supporters — indicating a scalable model for community worker involvement in disclosure counseling, which is key for adherence and
...
Treatment and care in children and adolescents
recommended
WHO recommends that infants born to mothers living with HIV are tested for HIV by two months of age, during breastfeeding, and when breastfeeding ends given continued risk of transmission during this period. Older children, especially offspring and siblings of persons infected with HIV, should also
...
Panels Recommendation Updated Dec 2024
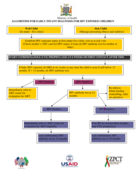
If baby HIV exposed, do DBS at six weeks or any time the child is seen if still below 12 months. If > 12 months, do HIV antibody test.
HIV rapid diagnostic test market landscape
recommended
The analysis includes the three most commonly used HIV rapid diagnostic test
(RDT) categories: HIV-only professional use RDTs, dual HIV/syphilis professional use
RDTs, and HIV self-tests (HIVST).
Orientation slides for trainers
Point of care EID training package
recommended
This training package provides materials to guide onsite training for the introduction of POC EID testing. The topics focus on what health workers at each facility need to learn in order to confidently begin POC EID high-quality testing; the package provides materials for both classroom and hands-on
...
A post-hoc instrumental variable-based analysis of a cluster randomized trial in Eldoret, Kenya. Front. Public Health, 05 May 2023
Sec. Public Mental Health Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1150744
Front. Public Health, 01 December 2023 Sec. Infectious Diseases: Epidemiology and Prevention Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1165557
Currently, there are only two manufacturers with HIV POC diagnostic products prequalified by the World Health
Organization (WHO) and eligible for procurement through the United Nations. UNICEF concluded its last tender for
HIV EID and VL POC diagnostic technologies in 2018 and awarded two manufact
...
Accessed: 26.10.2019
Policy and practice for maturity-aligned engagement of children in decisions about HIV-related medical and social services and management of confidential information