Filter
99
Text search:
remdesivir
Featured
Recommendations
31
New Publications
6
Language
Document type
Guidelines
55
Fact sheets
14
Studies & Reports
10
No document type
7
Manuals
4
Training Material
3
Resource Platforms
3
Infographics
1
Videos
1
Strategic & Response Plan
1
Countries / Regions
Germany
10
Brazil
8
Latin America and the Carribbean
8
Mozambique
6
Angola
6
Guinea-Bissau
4
Uganda
4
India
4
Rwanda
4
Congo, Democratic Republic of
3
South Sudan
3
Liberia
2
China
2
South Africa
2
Peru
2
Ecuador
2
Paraguay
2
Russia
2
Global
2
Spain
2
Kenya
1
Tanzania
1
Thailand
1
Argentina
1
Austria
1
Switzerland
1
Ukraine
1
Chile
1
East and Southern Africa
1
Africa
1
France
1
Authors & Publishers
Publication Years
Category
Countries
53
Pharmacy & Technologies
2
Public Health
1
Clinical Guidelines
1
Women & Child Health
1
Key Resources
1
Toolboxes
COVID-19
79
Ebola & Marburg
10
Pharmacy
5
Conflict
1
HIV
1
Remdesivir for COVID-19
recommended
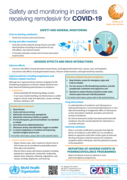
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
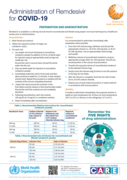
Administration of Remdesivir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
The purpose of this document is to stop to irrational use/ over prescription of this reserve/
experimental/ emergency use authorisation drug Remdesivir. For this reason, Joint
Monitoring Group under Chairmanship of DGHS too
...
This twelfth version of the WHO living guideline now contains 19 recommendations. This latest update provides updated recommendations for remdesivir, addresses the use of combination therapy with corticosteroids, interleukin-6 (IL-6) receptor blocke
...
A living WHO guideline on drugs for covid-19
recommended
BMJ 2020; 370 doi: https://doi.org/10.1136/bmj.m3379
Rapid Recommendation and visual graph. This is the fifth version (update 4) of the living guideline (BMJ 2020;370:m3379). When citing this article, please consider adding the update number and date of access for clarity. The publication of the RE
...
If you have COVID-19 and are caring for someone or yourself at home, what is the treatment protocol? What is WHO’s guidance on Remdesivir and convalescent plasma therapy? How to monitor oxygen at home and what are the red flags when you should cal
...
WHO has updated its guidelines for COVID-19 therapeutics, with revised recommendations for patients with non-severe COVID-19. This is the 13th update to these guidelines.
Updated risk rates for hospital admission in patients with non-severe COVID-19
The guidance includes updated risk rates for
...
3 March 2022
The WHO Therapeutics and COVID-19: living guideline contains the Organization’s most up-to-date recommendations for the use of therapeutics in the treatment of COVID-19. The latest version of this living guideline is available in pdf format (via the ‘Download’ button) and via an
...
Guía de tratamiento farmacológico y manejo deescenarios clínicos de casos de COVID-19 – enero 2022 Guía de tratamiento farmacológico y manejo deLeer más
16.7.2021
As of 31 October 2020 This is the tenth edition of this summary of rapid systematic reviews, which includes the results of a rapid systematic review of currently available literature. More than 200 therapeutic options or their combinations are being investigated in more than 1,700 clinical trials. I
...
O Centro Africano de Prevenção e Luta contra Doenças (CDC-África) está preocupado com a divulgação de informações incorrectas através de meios de comunicação tradicionais e sociais sobre a prevenção e o tratamento de nova doença de coronavírus (COVID-19).
16.7.2021
These forms are intended only for clinicians and nurses taking care of patients with Ebola virus disease. They provide standardized information that needs to be collected by the clinicians at admission time, every day and at time of discharge.
Updated Treatment Guidelines
Erfahrungen im Umgang mit COVID-19-Erkrankten–Hinweise von Klinikern für Kliniker –
Março de 2020
O número de Estados Membros da União Africana a reportarem
casos do COVID-19 está a aumentar e existe uma probabilidade de transmissão comunitária. A OMS modificou recentemente a definição
de caso suspeito do COVID-19, de modo a incluir infecção respiratória aguda grave
...
26 de abril del 2022. La Organización Panamericana de la Salud presenta estas consideraciones con el fin de apoyar la toma de decisiones relativa al manejo de pacientes con COVID-19 en la Región de las Américas. Las recomendaciones tienen en cuenta la evidencia más reciente, el estado de vacunac
...