Filter
14978
Filtered Results: 14978
Text search:
internet
Featured
Recommendations
1159
New Publications
3715
Language
Document type
No document type
8235
Studies & Reports
2604
Guidelines
1751
Manuals
702
Strategic & Response Plan
509
Fact sheets
424
Training Material
268
Situation Updates
177
Resource Platforms
105
Infographics
79
Brochures
76
Online Courses
20
Videos
17
Dashboards/Maps
7
App
4
Countries / Regions
Global
624
India
410
Kenya
355
Germany
328
Sierra Leone
261
Nigeria
256
Latin America and the Carribbean
254
Congo, Democratic Republic of
253
Ethiopia
251
Nepal
246
Western and Central Europe
243
South Africa
236
Myanmar / Burma
235
Liberia
234
Uganda
212
Senegal
199
Africa
195
Syria
191
Ukraine
189
Rwanda
181
Bangladesh
178
Zambia
168
Malawi
166
Tanzania
159
Indonesia
159
Burkina Faso
155
Brazil
140
Ghana
139
Mozambique
130
Russia
124
Guinea
123
South Sudan
118
Venezuela
117
Haiti
115
Eastern Europe
112
Philippines
106
Yemen
99
Benin
96
Zimbabwe
91
Namibia
91
Cameroon
81
Cambodia
81
West and Central Africa
81
Lesotho
80
Colombia
79
South–East Asia Region
73
Mali
73
Madagascar
70
Asia
69
East and Southern Africa
68
Middle East and North Africa
67
Central African Republic
65
Afghanistan
50
Eastern Europe and Central Asia
50
Côte d’Ivoire / Ivory Coast
50
Paraguay
47
Botswana
47
Pakistan
44
Peru
43
Argentina
43
Niger
39
Angola
37
Ecuador
37
Lebanon
36
Sudan
35
Eswatini/ Swaziland
34
Chile
32
Somalia
32
Jordan
30
Thailand
29
Bolivia
28
Chad
27
Iraq
27
Tajikistan
26
China
26
Vietnam
26
Guinea-Bissau
24
Western Pacific Region
24
Togo
23
USA
20
Spain
20
Albania
18
Moldova
18
Burundi
17
El Salvador
16
Turkey
16
North America
15
Sri Lanka
15
Palestine
14
Libya
14
Laos
12
Georgia
12
North Macedonia
11
Mexico
11
Kazakhstan
11
Iran
11
Kyrgyzstan
11
Portugal
11
Egypt
11
Greece
11
Switzerland
10
Armenia
9
Papua New Guinea
9
Morocco
8
France
8
Southern Africa
8
Romania
8
Guatemala
8
Serbia
7
Estonia
7
Uzbekistan
7
Malaysia
7
Italy
7
Saudi Arabia
7
Jamaica
7
Poland
7
Honduras
7
Timor Leste/ East Timor
6
Canada
6
Hungary
6
Turkmenistan
6
Japan
6
Tunisia
6
Djibouti
6
Gambia
5
Austria
5
Dominican Republic
5
Luxembourg
5
United Kingdom
5
Belarus
5
Bhutan
5
Fiji
4
Singapore
4
Congo-Brazzaville
4
Nicaragua
4
North Korea
3
Qatar
3
Mauritania
3
Croatia
3
Gabon
3
Uruguay
3
Belgium
3
Mauritius
3
Israel
3
Bosnia and Herzegovina
3
Australia
2
Vanuatu
2
Mongolia
2
Bulgaria
2
Lithuania
2
Algeria
2
Azerbaijan
2
Slovakia
2
Cape Verde
1
Ireland
1
Denmark
1
Maldives
1
Norway
1
South Korea
1
French Guyana
1
Latvia
1
Barbados
1
Solomon Islands
1
Cuba
1
Authors & Publishers
Publication Years
Category
Countries
5484
Clinical Guidelines
878
Key Resources
821
Public Health
693
Women & Child Health
690
Capacity Building
257
Pharmacy & Technologies
123
Annual Report MEDBOX
3
Toolboxes
COVID-19
1549
Mental Health
1208
HIV
891
TB
690
Disability
646
Refugee
580
Planetary Health
531
Ebola & Marburg
501
Conflict
466
Rapid Response
434
NTDs
405
Caregiver
375
AMR
332
Global Health Education
266
NCDs
259
Natural Hazards
237
Pharmacy
219
Health Financing Toolbox
205
Zika
133
Cholera
102
Polio
91
Specific Hazards
87
Social Ethics
86
Malaria
16
Typhoon
13
South Sudan
2
PQDx 0006-005-00 WHO
PQDx PR
February/2016, version 2.0
The technical note from the Global Task Force on Cholera Control (GTFCC) examines the risks and benefits of vaccinating pregnant women with WHO-prequalified oral cholera vaccines (OCVs) during mass vaccination campaigns. It highlights that three WHO-approved vaccines (Dukoral®, Shanchol™, and Euv...
December 2020
Рекомендации ВОЗ по оказанию дородовой помощи для формирования положительного опыта беременности
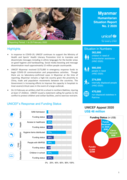
In response to COVID-19, UNICEF continues to support the Ministry of Health and Sports’ Health Literacy Promotion Unit to translate and disseminate messages including in ethnic languages for the border areas on good hygiene and handwashing. Social media boosting and message dissemination reach app...
Essential Medicines are those that satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy, safety and comparative cost-effectiveness. This edition of the Essential Medicines List (EML) 2017 for Ghana has been deriv...
China and Eurasia Forum Quarterly, Volume 6, No. 3 (2008) p. 101-128 © Central Asia-Caucasus Institute & Silk Road Studies Program
ISSN: 1653-4212
20 February 2013
Update on 2004 Background Paper (Written by Saloni Tanna)
Priority Medicines for Europe and the World "A Public Health Approach to Innovation"
31 Janaury 2021
SCORE for health data technical package. The first global assessment on the status and capacity of health information systems in 133 countries, covering 87% of the global population.
It identifies gaps and provides guidance for investment in areas that can have the greatest impact ...
This assessment tool is to support municipalities and local authorities in identifying the risks and vulnerabilities that refugees and migrants face and to identify gaps where possible methods to minimize the impact of the pandemic exist so that they can be prioritized within local policy processes.
Protection from a Single Dose of HPV Vaccine A major public health impact from IARC studies of vaccine efficacy.
23 February 2022
A summary of the commitments and targets within the United Nations General Assembly’s 2021 Political Declaration on HIV and AIDS.The United Nations General Assembly’s 2021 Political Declaration on AIDS features bold global commitments and targets for 2025 that are ambitious but...