Recommendations for Preparing Disinfecting Solution in Health Establishments
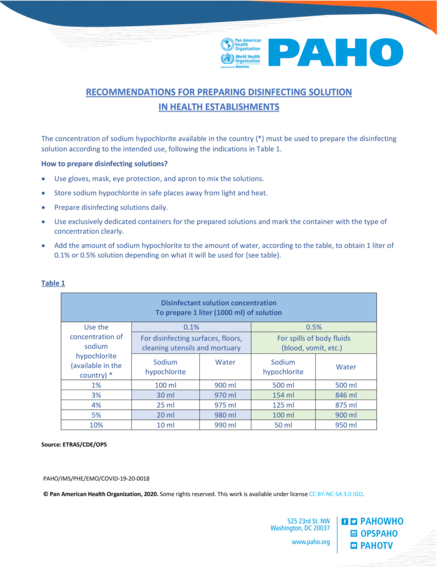
The concentration of sodium hypochlorite available in the country must be used to prepare the disinfecting solution according to the intended use, following the indications presented in this document.