Filter
1369
Text search:
specimen
Featured
221
409
Language
Document type
708
323
136
92
41
32
21
9
3
2
2
Countries / Regions
114
61
46
45
41
36
35
33
28
27
26
24
21
19
19
18
18
16
16
15
14
13
13
12
11
10
8
8
7
7
6
6
5
5
5
5
4
4
4
4
4
4
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
623
169
71
43
36
25
20
Toolboxes
186
151
80
78
69
69
49
43
23
21
18
17
17
16
15
9
9
7
5
5
4
3
1
1
The modules (1-12) are based on materials originally developed by FIND, KNCV and Cepheid, and are in PowerPoint format for country customization. Depending on the audience, modules may be selected and adapted according to need (e.g. basic users, supervisors, clinicians). Topics covered include: Over
...
Комплект учебных материалов по Xpert MTB/RIF
The modules (1-12) are based on materials originally developed by FIND, KNCV and Cepheid, and are in PowerPoint format for country customization. Depending on the audience, modules may be selected and adapted according to nee
...
Version 1.1 July 2016
The purpose of this document is to describe standard operating procedures for viral load monitoring, including the schedule for viral load testing when used for routine monitoring of children, adolescents and adults on ART; interpretation of results; patient management; an ...
The purpose of this document is to describe standard operating procedures for viral load monitoring, including the schedule for viral load testing when used for routine monitoring of children, adolescents and adults on ART; interpretation of results; patient management; an ...
The National Guidelines for HIV-1 Viral Load Laboratory Testing support plans to scale up viral load (VL) testing to reach the 90-90-90 targets in India. This phased scale-up includes the setup of 70 additional VL testing laboratories nationally. These guidelines include laboratory design considerat
...
The guide is presented in two parts:
Part 1. Principles of Operational Monitoring: Describes the key principles of operational monitoring, alongside the types of operational monitoring that may be performed and the information required within an OMP.
Part 2. Operational Monitorin ... Describes the stepwise development of an OMP for a water supply system, including the source, water treatment, intermediate storage, distribution and household. For illustration purposes, practical guidance is provided using a specimen water supply system considered to be representative of a conventional small- to medium-sized supply in a lower resource setting. This template may be used to develop system-specific OMPs for individual water supply systems.
Part 1. Principles of Operational Monitoring: Describes the key principles of operational monitoring, alongside the types of operational monitoring that may be performed and the information required within an OMP.
Part 2. Operational Monitorin ... Describes the stepwise development of an OMP for a water supply system, including the source, water treatment, intermediate storage, distribution and household. For illustration purposes, practical guidance is provided using a specimen water supply system considered to be representative of a conventional small- to medium-sized supply in a lower resource setting. This template may be used to develop system-specific OMPs for individual water supply systems.
In this document, recommendations are provided on designing and implementing
a cross-sectional serosurvey using school-based sampling to estimate age-specific
DENV seroprevalence to inform a country’s national dengue vaccination program.
The document includes recommendations for methods for
...
The Practical manual on laboratory strengthening, 2022 update provides practical guidance on implementation of WHO recommendations and best practices for TB laboratory strengthening. It is an updated version of the GLI Practical Guide to Laboratory Strengthening published in 2017 and provides the la
...
This updated manual provides a basic understanding of the principles of laboratory and point-of-care (POC) testing in the context of screening and diagnostic approaches, as well as antimicrobial susceptibility testing, as components of sexually transmitted infections (STIs) control. As with previous
...
This document provides technical guidance for manufacturers seeking World Health Organization (WHO) prequalification of in vitro diagnostic devices (IVDs) for malaria, with a focus on rapid diagnostic tests (RDTs) for symptomatic patients. It summarises the minimum performance requirements, includin
...
This document provides technical guidance for manufacturers seeking World Health Organization (WHO) prequalification of in vitro diagnostic devices (IVDs) for malaria, with a focus on rapid diagnostic tests (RDTs) for symptomatic patients. It summarises the minimum performance requirements, includin
...
The WHO publication “Surveillance, case investigation and contact tracing for mpox: interim guidance” provides updated global technical guidance on monitoring and responding to mpox (formerly known as monkeypox). It explains how countries should conduct surveillance to detect new outbreaks, car
...
The main objectives of these guidelines are to:
1. contribute to the quality assurance of medicinal plant materials used as the source for herbal medicines to improve the quality, safety and efficacy of finished herbal products; 2. guide the formulation of national and/or regional GACP guideli ...
1. contribute to the quality assurance of medicinal plant materials used as the source for herbal medicines to improve the quality, safety and efficacy of finished herbal products; 2. guide the formulation of national and/or regional GACP guideli ...
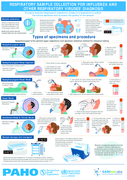
Respiratory sample collection for Influenza and other respiratory viruses diagnosis - Infographic
District hospital level Severe | Malaria is a Medical Emergency
Due to the anticipated significant rise in VL testing occasioned by Ghana’s adaptation of 2016 ART guidelines, it has become necessary to develop this VL scale-up and operational plan to assure complete client access to laboratory monitoring towards the achievement of the third 90 of the HIV care
...
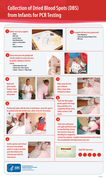
This clinical job aid provides health care workers with information on how to collect specimens for early infant diagnosis on dried blood spots, as well as drying and packaging for transport.
Household transmission investigation protocol for 2019-novel coronavirus (2019-nCoV) infection
recommended
The household transmission investigation is a case-ascertained prospective study of all identified household contacts of a laboratory confirmed 2019-nCoV infection (see 2.2 Study population). It is intended to provide rapid and early information on the clinical, epidemiological and virological chara
...
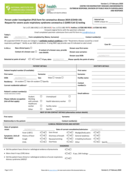
Person under investigation (PUI) form for coronavirus disease 2019 (COVID-19): Request for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing
and
COVID-19 Contact line list