Filter
173
Text search:
monoclonal
antibodies
Featured
30
30
Language
Document type
56
49
29
17
8
5
3
3
2
1
Countries / Regions
17
14
6
4
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
41
30
14
9
6
2
2
Toolboxes
53
13
11
9
9
6
5
5
4
3
3
3
3
3
2
1
1
1
1
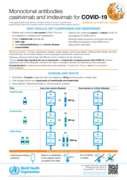
Poster, 4 Janaury 2022
Accessed Nov. 28,2016
May 9, 2022.Since the onset of the COVID-19 pandemic, a large number of clinical trials have been planned and developed to assess the effectiveness and safety of various interventions that could prevent hospitalizations and progression to severe disease in people infected with SARS-CoV-2. Currently,
...
The meeting was held from 26 to 27 March 2018 to review and discuss the following topics:
Advances and challenges in the use of fTLC, and new approaches to detecting mycolactone using monoclonal antib
...
This twelfth version of the WHO living guideline now contains 19 recommendations. This latest update provides updated recommendations for remdesivir, addresses the use of combination therapy with corticosteroids, interleukin-6 (IL-6) receptor blockers and Janus kinase (JAK) inhibitors in patients wi
...
The article reviews the impact of respiratory syncytial virus (RSV) on global health, emphasizing its significant burden on infants, children, and the elderly. It discusses current and emerging prevention strategies, including the development and implementation of vaccines and
...
Leishmaniasis is a vector-borne disease that is transmitted by sandflies and caused by obligate intracellular protozoa of the genus Leishmania. Human infection is caused by about 21 of 30 species that infect mammals. These include the L. donovani complex with 3 species (L. donovani, L. infantum, and
...
Therapeutics for Ebola virus disease
recommended
The WHO Ebola Virus Disease (EVD) Clinical management: living guidance contains the Organization’s most up-to-date recommendations for the clinical management of people with EVD. Providing guidance that is comprehensive and holistic for the optimal care of patients with EVD throughout their il
...
16.7.2021
Updated Treatment Guidelines
2016 ASCO EDUCATIONAL BOOK | asco.org/edbo
The COVID-19 Vaccine (Whole Virion Inactivated) BBV152, COVAXIN® vaccine explainer includes key information on the vaccine specific requirements.
New England Journal of Medicine
April 9, 2021
DOI: 10.1056/NEJMoa2104840
Clinical Infectious Diseases 2010; 50:291–322
3 March 2016
The Interim Guidance on Cholera Rapid Diagnostic Tests (RDTs) by the Global Task Force on Cholera Control (GTFCC) provides recommendations for using RDTs to detect cholera in areas with limited laboratory capacity. It highlights the advantages of RDTs, such as rapid detection (within 30 minutes), ea
...
sthma prevalence is increasing worldwide, and surveys indicate that most patients in developed and developing countries, including South Africa, do not receive optimal care and are therefore not well controlled. Standard management guidelines adapted to in-country realities are important to support
...