Filter
2230
Filtered Results: 2230
Text search:
specimen
Featured
Recommendations
308
New Publications
637
Language
Document type
No document type
1264
Guidelines
450
Studies & Reports
219
Manuals
115
Strategic & Response Plan
71
Fact sheets
53
Training Material
26
Situation Updates
18
Infographics
6
Videos
3
Online Courses
2
Brochures
1
Dashboards/Maps
1
Resource Platforms
1
Countries / Regions
India
146
South Africa
76
Nigeria
64
Kenya
61
Liberia
59
Sierra Leone
55
Western and Central Europe
50
Global
42
Ethiopia
42
Africa
38
Tanzania
38
Ghana
36
Zambia
35
Rwanda
34
Congo, Democratic Republic of
34
Uganda
31
Malawi
29
Nepal
24
Indonesia
24
Latin America and the Carribbean
23
Namibia
23
Zimbabwe
22
Philippines
22
West and Central Africa
22
Eastern Europe
21
Bangladesh
20
East and Southern Africa
18
Russia
18
Lesotho
17
Guinea
16
Myanmar / Burma
15
Germany
13
Syria
12
Haiti
12
Cambodia
12
Mozambique
11
Eastern Europe and Central Asia
11
Middle East and North Africa
10
Senegal
9
Burkina Faso
9
South–East Asia Region
9
Ukraine
8
Brazil
8
Botswana
8
Madagascar
8
Eswatini/ Swaziland
7
Tajikistan
6
Asia
6
Western Pacific Region
6
Spain
6
Mali
6
South Sudan
6
Pakistan
6
Benin
5
Cameroon
5
Peru
5
Angola
5
Afghanistan
4
Italy
4
USA
4
Côte d’Ivoire / Ivory Coast
4
Somalia
4
Colombia
3
Turkey
3
China
3
France
3
Argentina
3
Vietnam
3
Yemen
3
Palestine
3
Chad
3
Thailand
2
Gambia
2
Togo
2
Burundi
2
Qatar
2
Guinea-Bissau
2
Jordan
2
Saudi Arabia
2
Paraguay
2
Gabon
2
Southern Africa
2
Bolivia
2
Lebanon
2
Central African Republic
2
Libya
2
Iraq
2
North Macedonia
1
Timor Leste/ East Timor
1
El Salvador
1
Australia
1
Iran
1
Laos
1
Uzbekistan
1
Morocco
1
Mauritania
1
Venezuela
1
North America
1
Uruguay
1
Maldives
1
Mauritius
1
United Kingdom
1
Portugal
1
Moldova
1
Sri Lanka
1
Djibouti
1
Egypt
1
Sudan
1
Romania
1
Georgia
1
Ecuador
1
Authors & Publishers
Publication Years
Category
Countries
936
Clinical Guidelines
260
Pharmacy & Technologies
89
Public Health
77
Key Resources
59
Women & Child Health
41
Capacity Building
23
Toolboxes
COVID-19
282
TB
266
Rapid Response
190
HIV
169
Ebola & Marburg
124
AMR
114
NTDs
107
Pharmacy
36
Caregiver
35
Zika
35
Polio
28
Mental Health
27
Cholera
27
Planetary Health
21
Natural Hazards
17
Refugee
16
Conflict
12
Specific Hazards
10
NCDs
6
Health Financing Toolbox
4
Global Health Education
3
Disability
1
This resource consists of technical guidelines for District Medical Officers, counselors and laboratory technicians for second-line antiretroviral therapy drugs, operational guidelines for pilot roll-out in two centres and laboratory guidelines for viral-load testing and standard operating procedure...
The GTFCC Laboratory Support for Public Health Surveillance document provides guidelines on using DNA-based molecular techniques for identifying and monitoring Vibrio cholerae strains in cholera outbreaks. It highlights the importance of genetic sequencing for tracking transmission, detecting new va...
Circulating vaccine-derived poliovirus type 2 in Angola
Ebola virus disease in Democratic Republic of the Congo
Dengue fever in Côte d’Ivoire
Humanitarian crisis in north-east Nigeria.
Ebola Virus Disease Outbreak Response Plan in West Africa July to December 2014. Annex 1
Morbidity and Mortality Weekly Report (MMWR) Early Release Vol. 64 ; 1 May 2015
Federal Bureau of Prisons
Clinical Practice Guidelines
January 1010
These forms are intended only for clinicians and nurses taking care of patients with Ebola virus disease. They provide standardized information that needs to be collected by the clinicians at admission time, every day and at time of discharge.
This manual describes some of the strategic, managerial, financial, technical and scientific aspects to be considered in establishing a national EQA programme for clinical laboratories and other testing services at all health care levels
30 April 2020
| COVID-19: Essential health services
Testing Guidance and Interpretation of Results for Healthcare Providers
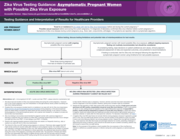
Zika Virus Testing Guidance: Asymptomatic Pregnant Women
with Possible Zika Virus Exposure
Detection, Confirmation and Management of a Dysentery Outbreak caused by Shigella Dysenteriae type 1
Standard Operating Procedures | RBC/IHDPC/EID Division | 9/30/2011
July 2016
PQDx 0033-013-00 WHO
PQ Public Report
July/2016, version 5.0