Filter
5276
Filtered Results: 5276
Text search:
Respiratory
Featured
Recommendations
645
New Publications
1083
Language
Document type
No document type
2170
Guidelines
1194
Studies & Reports
877
Manuals
297
Fact sheets
238
Strategic & Response Plan
187
Training Material
93
Situation Updates
65
Infographics
55
Resource Platforms
30
Online Courses
26
Brochures
19
Videos
13
Dashboards/Maps
11
App
1
Countries / Regions
Global
307
India
263
Kenya
146
South Africa
127
Latin America and the Carribbean
116
Western and Central Europe
104
Africa
96
Nepal
90
Nigeria
85
Ethiopia
82
Brazil
79
Rwanda
76
Bangladesh
73
Liberia
73
Sierra Leone
72
Uganda
69
Ghana
65
Syria
60
Philippines
58
Colombia
57
Russia
56
Malawi
53
Myanmar / Burma
53
Zambia
52
Germany
51
Paraguay
49
Tanzania
48
West and Central Africa
48
Mozambique
47
Namibia
47
East and Southern Africa
43
Argentina
43
Middle East and North Africa
42
South–East Asia Region
36
Eastern Europe
35
Ukraine
32
Peru
31
Venezuela
31
Ecuador
30
Senegal
29
Indonesia
29
Eastern Europe and Central Asia
28
Lesotho
27
Congo, Democratic Republic of
27
Chile
25
Haiti
25
Cambodia
25
Angola
25
Yemen
24
Zimbabwe
23
Bolivia
23
El Salvador
22
Guinea-Bissau
21
Guinea
20
Spain
20
South Sudan
19
Burkina Faso
18
Asia
18
Western Pacific Region
16
Eswatini/ Swaziland
16
USA
14
Mali
13
Botswana
13
Madagascar
12
Pakistan
12
Thailand
11
Tajikistan
10
Cameroon
10
Italy
10
North America
10
Vietnam
10
Jordan
9
Sudan
9
Iraq
9
China
8
Afghanistan
8
Côte d’Ivoire / Ivory Coast
8
Papua New Guinea
8
Mexico
7
Turkey
7
Benin
7
Saudi Arabia
6
United Kingdom
6
Lebanon
6
Somalia
6
Albania
5
Laos
5
Kyrgyzstan
5
Niger
5
Ireland
5
Portugal
5
Central African Republic
5
Timor Leste/ East Timor
4
Kazakhstan
4
France
4
Moldova
4
Georgia
4
Honduras
4
Chad
4
Libya
4
North Macedonia
3
Switzerland
3
Iran
3
Dominican Republic
3
Southern Africa
3
Nicaragua
3
Sri Lanka
3
Egypt
3
Greece
3
Palestine
3
Bhutan
3
Guatemala
3
Serbia
2
Singapore
2
Uzbekistan
2
Burundi
2
Malaysia
2
Morocco
2
Qatar
2
Bulgaria
2
Mauritius
2
Tunisia
2
Romania
2
Australia
1
Gambia
1
Canada
1
Fiji
1
Estonia
1
Congo-Brazzaville
1
Austria
1
Mongolia
1
Mauritania
1
Armenia
1
Hungary
1
Uruguay
1
Turkmenistan
1
Japan
1
Denmark
1
Maldives
1
Azerbaijan
1
Belarus
1
Israel
1
Bosnia and Herzegovina
1
Latvia
1
Djibouti
1
Slovakia
1
Poland
1
Cuba
1
Authors & Publishers
Publication Years
Category
Countries
2321
Clinical Guidelines
427
Public Health
379
Key Resources
225
Women & Child Health
218
Capacity Building
95
Pharmacy & Technologies
64
Toolboxes
COVID-19
1224
TB
432
NCDs
267
Planetary Health
202
Caregiver
170
Mental Health
168
AMR
159
Rapid Response
139
Ebola & Marburg
130
HIV
126
Conflict
95
Pharmacy
91
NTDs
82
Global Health Education
78
2.0 Rapid Response
70
Refugee
64
Natural Hazards
53
Specific Hazards
49
Disability
43
Health Financing Toolbox
28
Malaria
28
Zika
27
Cholera
25
Polio
22
Social Ethics
2
Typhoon
2
In the light of the transmissibility of coronaviruses, and the global experience with MERS-CoV (ongoing) and SARS in 2003 which were also caused by coronaviruses, South African authorities have compiled this guideline document to support surveillance, case finding, diagnosis, management and public h...
Lancet Respir Med 2020Published OnlineMarch 20, 2020 https://doi.org/10.1016/S2213-2600(20)30134-
Accessed: 08.04.2020
Fact sheet on Tuberculosis in Rwanda
Updated 24 March 2020
COVID-19, General Epidemiological Surveillance Guidelines (Version 6)
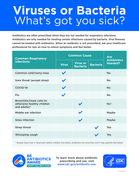
Antibiotics are only needed for treating certain infections caused by bacteria. Viral illnesses cannot be treated with antibiotics. When an antibiotic is not prescribed, ask your healthcare professional for tips on how to relieve symptoms and feel better.
29 September 2021
Updated 24 March 2020
Updated 24 March 2020
The Government of India is embarking on a mammoth task to prevent COVID-19 spread among communities. The Rapid Evidence Synthesis team received a request to support the planning and development of resources for ensuring preparedness of FLHWs for COVID-19 . The rapid evidence synthesis was conducted ...
This brief summarizes current evidence and guidance for maintaining safe and effective care across the spectrum of maternal, newborn and infant care while protecting mother and child and health care providers during COVID-19. Furthermore, implications of the principle of “do no harm” are reviewe...
Journal of Infectious Diseases and Therapeutics, 2013, 1, 17-24
BMJ 2020; 370 doi: https://doi.org/10.1136/bmj.m3026 (Published 11 August 2020)
The BMJ "practice pointer" inlcudes a one-page visual summary of assessment and initial management of patients with persistant symptoms following acute SARS-CoV-2 infection
(7th Version)
20, March 2020
Effective implementation of WHO PEN, combined with other very cost effective population-wide interventions, will help even resource constrained settings to attain the global voluntary targets related to reduction of premature mortality and preventionof heart attacks and strokes.
The purpose of this document is to provide interim guidance to laboratories and stakeholders involved in laboratory testing of patients who meet the definition of suspected case of pneumonia associated with a novel coronavirus identified in Wuhan, China.
19 March 2020