Filter
26
Featured
14
5
Language
Document type
12
9
2
2
1
Countries / Regions
3
3
2
2
2
1
1
1
1
1
1
Authors & Publishers
Publication Years
Toolboxes
13
2
2
2
1
1
1
Updated recommendations on simplified service delivery and diagnostics for hepatitis C infection
recommended
Policy Brief. 24 June 2022. This policy brief, one of two on the updated hepatitis C (HCV) guidelines, focuses on the new recommendations on simplified service delivery for a public health approach to HCV testing, care and treatment. These recommendations include decentralization, integration and ta
...
To meet the need for laboratory diagnosis to be integrated in community care, the new 2022 Update describes point-of-care tests that can be performed both in district laboratories and peripheral healthcare facilities by non-specialist staff. Also included are recently developed self-tests.
8 January 2021
Sequencing enabled the world to rapidly identify SARS-CoV-2 and develop diagnostic tests and other tools for outbreak management. Continued genome sequencing supports the monitoring of the disease’s spread and evolution of the virus. Accelerated integration of genome sequencing int
...
The growing understanding of how sequence information can contribute to improved public health is driving global investments in sequencing facilities and programmes. The falling cost and complexity of generating GSD provides opportunities for expanding sequencing capacity; however, challenges to wid
...
Ces orientations se concentrent sur le SARS-CoV-2, mais s'appliquent également à d'autres agents pathogènes préoccupants pour la santé publique.
The purpose of this document is to provide interim guidance to laboratories and stakeholders involved in laboratory testing of patients who meet the definition of suspected case of pneumonia associated with a novel coronavirus identified in Wuhan, China.
19 March 2020
Tests zur Detektion einer Infektion mit SARS-CoV-2 (neuartiges Coronavirus) sowie zum Nachweis von Antikörpern gehören zu den sog. In-vitro-Diagnostika (IVD). Das erstmalige Inverkehrbringen von In-vitro-Diagnostika auf dem deutschen Markt ist nach §§ 25 und 30 Medizinproduktegesetz (MPG) anzeig
...
The speed of developing diagnostics for SARS-CoV-2, the causative agent of coronavirus disease 2019 (COVID-19), has been quite remarkable. Diagnostics have focused on nucleic acid amplification testing (NAAT) to identify infected individuals in acute-phase disease for timely implementation of mitiga
...
8 July 2020
Direct acting antivirals (DAAs) have revolutionized treatment for hepatitis C. Combi-
nations of DAAs can cure infection with HCV in 12 weeks, are highly effective and
have limited side-effects. Affordability of DAAs has improved significantly, but access remains lim-
i
...
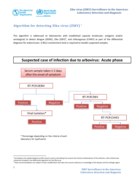
This algorithm is addressed to laboratories
with established capacity(molecular, antigenic and/orserological) to detect dengue (DENV), Zika (ZIKV), and chikungunya(CHIKV) as part of the differential diagnosis for arborviruses. A BSL2 containment level is required to handle suspected samples.
Este algoritmo está dirigido a aquellos laboratorios que cuentan con capacidad instalada para la detección (molecular, antigénica y/o serológica) de dengue (DENV), Zika (ZIKV) y chikungunya(CHIKV),y como parte del diagnóstico diferencial para Arbovirus. Para la manipulación de muestras sosp
...
July 2016