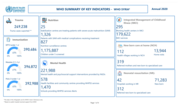
Filter
6546
Text search:
health
authorities
Featured
Recommendations
631
New Publications
1639
Language
Document type
No document type
3212
Studies & Reports
1138
Guidelines
962
Manuals
453
Strategic & Response Plan
317
Fact sheets
158
Training Material
124
Situation Updates
112
Resource Platforms
20
Infographics
17
Online Courses
14
Brochures
13
Dashboards/Maps
4
Videos
2
Countries / Regions
Global
319
India
201
Western and Central Europe
168
Liberia
135
Sierra Leone
134
Myanmar / Burma
123
Syria
114
Latin America and the Carribbean
112
Kenya
107
Nigeria
104
Ukraine
94
Uganda
93
Ethiopia
91
Congo, Democratic Republic of
89
Rwanda
86
Africa
81
Bangladesh
79
Nepal
76
Tanzania
67
South Africa
64
Malawi
64
Ghana
62
South Sudan
57
Zambia
57
South–East Asia Region
57
Guinea
55
Eastern Europe
52
Yemen
48
West and Central Africa
43
Mozambique
42
Namibia
40
Asia
40
Venezuela
40
Cambodia
39
Haiti
37
Zimbabwe
35
Senegal
34
East and Southern Africa
34
Lesotho
33
Germany
32
Eastern Europe and Central Asia
32
Middle East and North Africa
31
Indonesia
30
Philippines
29
Colombia
27
Burkina Faso
26
Central African Republic
26
Afghanistan
24
Brazil
24
Mali
22
Sudan
22
Cameroon
21
Pakistan
21
Russia
20
Lebanon
19
Botswana
19
Somalia
18
Jordan
18
Western Pacific Region
18
Vietnam
18
Iraq
16
Moldova
16
China
15
Madagascar
15
Côte d’Ivoire / Ivory Coast
14
Thailand
14
Laos
14
Niger
12
Eswatini/ Swaziland
12
Tajikistan
12
Benin
11
Palestine
11
Turkey
10
Peru
10
Chile
10
Chad
9
Iran
9
USA
8
Togo
8
Greece
8
Libya
8
Angola
8
Albania
8
North America
8
North Macedonia
7
Sri Lanka
7
Georgia
7
Guinea-Bissau
6
Egypt
6
Burundi
6
Ecuador
6
El Salvador
6
Mexico
6
Poland
6
Kyrgyzstan
6
Kazakhstan
6
Jamaica
6
Serbia
5
Bolivia
5
Italy
5
Timor Leste/ East Timor
5
Romania
5
Turkmenistan
5
Tunisia
5
Spain
5
Morocco
4
Saudi Arabia
4
Gambia
4
Papua New Guinea
4
Argentina
4
Hungary
4
Honduras
4
Guatemala
4
Nicaragua
4
Paraguay
4
United Kingdom
4
Portugal
4
Malaysia
3
Mauritania
3
Dominican Republic
3
Croatia
3
Fiji
3
Bhutan
3
Djibouti
3
Armenia
3
Southern Africa
3
Qatar
3
Uzbekistan
3
Israel
3
Bosnia and Herzegovina
3
Switzerland
2
Mongolia
2
Bulgaria
2
Estonia
2
Lithuania
2
Australia
2
Japan
2
France
2
Mauritius
2
Azerbaijan
2
Belarus
2
Singapore
1
Vanuatu
1
Austria
1
Cuba
1
Other region
1
North Korea
1
Congo-Brazzaville
1
Slovakia
1
Gabon
1
Denmark
1
Maldives
1
Norway
1
South Korea
1
Algeria
1
Morocco
1
French Guyana
1
Belize
1
Costa Rica
1
Panama
1
Authors & Publishers
Publication Years
Category
Countries
1959
Clinical Guidelines
527
Key Resources
510
Public Health
392
Women & Child Health
376
Capacity Building
132
Pharmacy & Technologies
101
Annual Report MEDBOX
1
Toolboxes
COVID-19
736
HIV
426
Mental Health
419
TB
322
Ebola & Marburg
289
Conflict
264
Planetary Health
233
Disability
225
NTDs
220
Rapid Response
194
Refugee
187
AMR
163
Caregiver
157
Natural Hazards
127
Pharmacy
127
Global Health Education
115
2.0 Rapid Response
106
Malaria
94
NCDs
78
Zika
74
Cholera
66
Health Financing Toolbox
61
Specific Hazards
59
Polio
49
Social Ethics
12
Typhoon
7
South Sudan
1
This document aims to provide concrete, pragmatic guidance for how TB modelling and related technical assistance is undertaken to support country decision-making. The target audience for this document are the participants and stakeholders in country-level TB modelling efforts, including the individu
...
Indoor residual spraying (IRS) involves applying residual insecticide to potential vector resting sites on the interior surfaces of human dwellings or other buildings. The main aim of IRS is to kill vectors before they are able to transmit pathogens to humans. When carried out correctly, IRS has his
...
Vector-borne diseases are responsible for 17% of the global burden of communicable diseases and more than 500 000 deaths annually. The ambitious global targets for the control of vector-borne diseases come in the context of the (re-)emergence of diseases, increasing resistances to insecticides and u
...
Of the 50 antibiotics in the pipeline, 32 target WHO priority pathogens but the majority have only limited benefits when compared to existing antibiotics. Two of these are active against the multi-drug resistant Gram-negative bacteria, which are spreading rapidly and require urgent solutions.
Gr
...
The first important change is a new priority ranking of the available medicines for MDR-TB treatment, based on a careful balance between expected benefits and harms. Treatment success for MDR-TB is currently low in many countries. This could be increased by improving access to the highest-ranked med
...
This protocol provides information on the safe management of dead bodies and burial of patients who died from suspected or confirmed Ebola virus disease.
These measures should be applied not only by medical personnel but by anyone involved in the management of dead bodies and burial of suspected o
...
Technical Update
Areas of Africa endemic for Buruli ulcer (BU), caused by Mycobacterium ulcerans, also have a high prevalence of human immunodeficiency virus (HIV), with adult prevalence rates between 1% and 5% (Maps). However, there is limited information on the prevalence of BU–HIV coinfection.
...
This Rapid Communication aims to inform national TB programmes and other stakeholders about the key implications of the latest evidence on the use of specific molecular assays as initial diagnostic tests of pulmonary and extrapulmonary TB and RR-TB, in adults and children.
The leishmaniases are a group of diseases caused by Leishmania spp., which occur in cutaneous, mucocutaneous and visceral forms. They are neglected tropical diseases (NTDs), which disproportionately affect marginalized populations who have limited access to
...
The purpose of cancer screening tests is to detect pre-cancer or early-stage cancer in asymptomatic individuals so that timely diagnosis and early treatment can be offered, where this treatment can lead to better outcomes for some people.
The aim of a cancer screening programme is either to reduc
...