Filter
10
Text search:
molnupiravir
Featured
Recommendations
1
Language
Document type
Studies & Reports
3
Guidelines
2
Strategic & Response Plan
2
Fact sheets
2
Resource Platforms
1
Countries / Regions
Ukraine
2
Global
2
Paraguay
1
Africa
1
Authors & Publishers
Publication Years
Category
Countries
4
Public Health
1
Key Resources
1
Pharmacy & Technologies
1
Toolboxes
COVID-19
8
Conflict
2
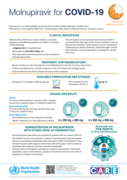
Molnupiravir for COVID-19
recommended
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
WHO has updated its guidelines for COVID-19 therapeutics, with revised recommendations for patients with non-severe COVID-19. This is the 13th update to these guidelines.
Updated risk rates for hospital admission in patients with non-severe COVID-19
The guidance includes updated risk rates for
...
Guía de tratamiento farmacológico y manejo deescenarios clínicos de casos de COVID-19 – enero 2022 Guía de tratamiento farmacológico y manejo deLeer más
The World Health Organization (WHO) recognizes the challenges countries face for maintaining their COVID-19 response while addressing competing public health challenges, conflicts, climate change and economic crises. WHO continues to support countries in adjusting COVID-19 strategies to reflect succ
...
This bi-weekly brief details the latest developments in scientific knowledge and public health policy from around the world as well as updates to the COVID-19-related guidance from Africa CDC, WHO and other public health agencies.
Saving lives is the priority of WHO’s response in Ukraine. WHO works to ensure time-critical, lifesaving multisectoral assistance, non-discriminatory access to emergency and essential health services and priority prevention programmes, and laying the foundation for longer-term health systems recov
...
The two-year impact report for the Access to COVID-19 Tools (ACT) Accelerator details impact, case studies and timelines of key milestones for the Diagnostics, Therapeutics and Vaccines pillars, as well as the Health Systems and Response Connector.
The military offensive by the Russian Federation in Ukraine which began February 2022 has triggered one of the world’s fastest-growing displacement and humanitarian crisis, with geopolitical and economic ripples felt across the globe. The ongoing war has caused large-scale disruptions to the deliv
...
31 Oct 2022 his plan outlines how the ACT-Accelerator will support countries as the world transitions to long-term COVID-19 control.
Recognizing the evolving nature of the COVID-19 virus and pandemic, the plan outlines changes to ACT-A’s set-up and ways of working, to ensure countries co
...
The second edition of the joint WHO, WIPO and WTO
publication “Promoting Access to Medical Technologies
and Innovation: Intersections between public health,
intellectual property and trade” (the Trilateral Study),*
published in 2020, included a special insert mappi
...