Filter
700
Text search:
candidates
Featured
65
172
Language
Document type
347
138
87
34
33
25
12
10
6
4
3
1
Countries / Regions
45
27
18
18
16
16
14
12
12
12
11
11
11
10
10
9
9
9
8
8
8
8
7
7
6
6
6
6
6
5
5
5
5
5
4
4
4
4
4
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
258
52
28
25
20
8
4
1
Toolboxes
111
40
39
35
32
27
24
21
16
13
12
9
9
8
8
7
7
6
6
6
4
4
4
3
3
The report presents current information (updated to September 2015) on candidate vaccines, therapies and medical devices for Ebola and gives an overview of completed and on-going trials.
August 2020, The Africa Joint Continental Strategy for COVID-19 is underpinned by the need to limit transmission, prevent deaths and reduce associated harms. Participation by African nations in clinical trials is an essential step to ensure that sufficient data is generated on the safety and efficac
...
Scientists and researchers managed to produce vaccines to protect against COVID-19. Vaccine candidates have recently been approved in some countries and are in the approval process in others, yet misinformation about the safety and effects of any fu
...
This tracker, developed by the Vaccine Centre at the London School of Hygiene & Tropical Medicine, will follow COVID-19 vaccine candidates as they progress through the development pipeline. We will update it weekly. An overview of the different vac
...
COVID-19 vaccine tracker and landscape
recommended
The COVID-19 vaccine tracker and landscape compiles detailed information of each COVID-19 vaccine candidate in development by closely monitoring their progress through the pipeline.
The COVID-19 vaccine tracker:
Provides summary tables of COVID-19 vaccine
...
BioDrugs. 2023 Sep 20;37(6):737–756. doi: 10.1007/s40259-023-00623-4
There are many malaria vaccine candidates in development, with more than a dozen of these in clinical development. RTS,S/AS01 (also known as Mosquirix) is the most advanced mal
...
10 December 2020
Comment The Lancet Volume 397, ISSUE 10269, P72-74, January 09, 2021
Published:December 08, 2020DOI:https://doi.org/10.1016/S0140-6736(20)32623-4
This article is part four in a series of explainers on vaccine development and distribution.
Part one focused on how vaccines work to protect our bodies from disease-carrying germs.
Part two focused on the ingredients in a vaccine and the three clinical trial phases.
Part three focused on the ste
...
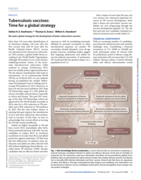
There is an urgent need for safer, simpler, more efficacious and accessible treatment regimens for all forms of TB. The development of Target Product Profiles for TB treatment regimens (referred to as Target Regimen Profiles or TRPs) seeks to guide the drug development process towards important regi
...
Policy
25 February 2015 Vol 7 Issue 276 276fs8
Will COVID-19 vaccines be safe? Will all the COVID-19 candidate vaccines be successful? What are the different phases a vaccine must go through to be approved? This document provides responses to the most frequently asked questions about candidate vaccines and access to COVID-19 vaccination.
COVID-19 & Vaccination
recommended
Issue Brief no.8. January 18,2021
Vaccination against the spread of the SARS-CoV-2 virus represents a milestone in the fight against the pandemic. Good communication and education of the population is essential for the success of the vaccination. Below is some information on vaccination strategies,
...
Published:February 02, 2021DOI:https://doi.org/10.1016/S0140-6736(21)00234-8
On the 9 February 2021, Africa CDC convened a special session of the Africa Task Force for COVID-19 to review existing data and evidence and recommend
How far apart should the doses of vaccines be? What if I miss my second dose? Can I get two doses from two different manufacturers? How was safety of vaccines ensured? WHO’s Chief Scientist, Dr Soumya Swaminathan explains in Science in 5.
New England Journal of Medicine 374:1,pp23-32