Filter
1509
Text search:
antimicrobial
Featured
189
296
Language
Document type
382
354
341
105
103
84
32
27
24
18
15
14
9
1
Countries / Regions
70
55
38
36
34
27
25
24
18
17
15
15
13
12
12
11
11
9
9
9
9
9
8
8
8
7
7
7
7
7
6
6
6
5
5
5
5
4
4
4
4
4
4
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
390
116
112
58
48
28
21
3
Toolboxes
555
102
92
86
66
62
47
46
37
37
35
26
25
25
17
14
14
10
9
6
5
3
2
1
The Global Health eLearning Center offers courses aimed at increasing knowledge in a variety of global health technical areas. Courses that have been translated and can be found on the Translation page.
Accessed 6 March 2019.
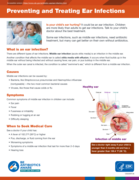
Is your child’s ear hurting? It could be an ear infection. Children are more likely than adults to get ear infections. Talk to your child’s doctor about the best treatment. Some ear infections, such as middle ear infections, need antibiotic treatment, but many can get better on their own without
...
List of available resources and courses
Accessed on 10.09.2022
Para evaluar la situación basal de los PROA en Chile, el grupo iniciativa del Milenio para la Investigación Colaborativa en Resistencia Bacteriana (MICROB-R), realizó una encuesta que en colaboración con el Ministerio de Salud debía ser respondida por los hospitales p�
...
The GEHM series is an evidence-informed normative product of the WHO
Health and Migration Programme to inform policy-makers on migrationrelated public health priorities. These reviews aim to respond to policy questions identified as priorities by summarizing the best available evidence worldwide an
...