Filter
5855
Text search:
tests
Featured
625
1390
Language
4820
915
153
130
101
76
47
31
13
9
9
9
8
6
6
6
6
5
5
5
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
Document type
2892
940
937
358
210
207
148
53
31
30
26
10
8
1
Countries / Regions
310
222
139
134
130
130
103
101
98
97
93
90
84
81
81
73
73
72
71
70
68
60
57
51
45
44
42
41
41
39
39
39
38
36
36
34
28
28
28
27
27
26
26
25
25
23
23
20
19
19
17
16
16
15
14
13
13
11
11
11
10
10
10
10
9
9
9
9
8
8
8
8
8
8
8
7
7
7
7
6
6
6
6
5
5
5
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
748
253
232
148
113
105
91
83
80
65
63
63
59
39
38
38
34
34
33
30
29
28
26
25
25
25
23
23
23
23
22
21
21
21
21
20
20
20
19
19
18
18
18
17
16
16
15
15
15
15
15
15
14
14
14
13
13
13
13
13
13
13
13
12
12
11
11
11
11
11
11
11
11
10
10
10
10
10
10
10
9
9
9
9
9
8
8
8
8
8
8
8
8
8
8
8
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
7
6
6
6
6
6
6
6
6
6
6
6
6
6
6
6
6
6
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Publication Years
1901
3416
503
33
2
Category
2318
520
288
242
222
146
129
2
Toolboxes
676
453
398
311
261
203
200
167
148
141
120
119
102
87
85
82
77
52
51
48
43
28
26
25
4
1
8 February 2021
This document highlights 10 well established Risk Communication and Community Engagement (RCCE) principles that have proven their power. Together they put communities at the heart of the roll out of new vaccines, treatments and tests
...
and promote trust, the critical ingredient for all community action. Informed, engaged and empowered communities are the bedrock for the arrival of new vaccines, treatments and tests that will be introduced to reduce the spread of COVID-19 and save lives. With communities fully engaged and actively participating through the full cycle of planning, delivery and assessment for biomedical tools, demand for these tools can be increased, leading to widespread and effective uptake and use
more
These guidelines provide new and updated recommendations on the use of point-of-care testing in children under 18 months of age and point-of-care tests to monitor treatment in people living with HIV; the treatment monitoring algorithm; and timing of
...
antiretroviral therapy (ART) among people living with HIV who are being treated for tuberculosis.
New recommendations launched today outline key new actions that countries can take to improve the delivery of HIV testing, treatment and care services by providing greater options for differentiated approaches such as, supporting HIV treatment start in the community, ensuring that children are diagnosed and treated early, and that viral load treatment monitoring is more accessible, focused and triggers clinical action
more
These guidelines provide new and updated recommendations on the use of point-of-care testing in children under 18 months of age and point-of-care tests to monitor treatment in people living with HIV; the treatment monitoring algorithm; and timing of
...
antiretroviral therapy (ART) among people living with HIV who are being treated for tuberculosis.
New recommendations launched today outline key new actions that countries can take to improve the delivery of HIV testing, treatment and care services by providing greater options for differentiated approaches such as, supporting HIV treatment start in the community, ensuring that children are diagnosed and treated early, and that viral load treatment monitoring is more accessible, focused and triggers clinical action
more
Systematic screening for TB disease is the systematic identification of people at risk for TB disease, in a predetermined target group, by assessing symptoms and using tests, examinations or other procedures that can be applied rapidly.
Systematic
...
screening can benefit people who are at risk of getting TB, as early detection and start of treatment can improve their outcomes and reduce their costs. It can also benefit entire communities at higher risk for TB, by reducing the prevalence of TB disease and preventing future cases.
more
Les infections au SRAS-CoV-2 chez les enfants et les adolescents provoquent une maladie moins grave et moins de décès que chez les adultes. Bien qu'une évolution moins grave de l'infection soit un résultat positif, on s'inquiète du fait que des symptômes légers aient pu conduire à moins de
...
tests, ce qui a entraîné un nombre moins important de cas identifiés de COVID-19 chez les enfants. Si les enfants présentant des symptômes légers ou inexistants transmettent la maladie, ils peuvent agir comme des vecteurs de transmission au sein de leur communauté. Il est essentiel de comprendre les symptômes, l'infectivité et les modes de transmission du SRAS-CoV-2 chez les enfants et les adolescents pour développer, adapter et améliorer les mesures de contrôle du COVID-19 à tous les âges. Ce document est un résumé des connaissances actuelles concernant l'acquisition et la transmission de l'infection par le SRAS-CoV-2 et les symptômes de la maladie COVID-19 chez les enfants et les adolescents. Il vise à éclairer les décisions, en fonction des contextes locaux, sur la meilleure façon de maintenir ouvertes les écoles, les jardins d'enfants et les crèches et sur les conseils à appliquer en matière de mixité intergénérationnelle.
more
Über SARS-CoV-2-Testsysteme informieren das Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) und das Paul-Ehrlich-Institut. Die Informationen ergänzen sich, den rechtlichen Rahmen bietet u.a. die Coronavirus-Testverordnung-TestV.
Das BfArM bietet eine Liste von Antigen-
...
Tests zum direkten Erregernachweis des Coronavirus SARS-CoV-2 an. In dieser Liste befinden sich diejenigen Tests, die sich laut Herstellerangaben gemäß den Vorgaben des Medizinproduktegesetzes (MPG) rechtmäßig in Europa bzw. Deutschland in Verkehr befinden und alle vom Paul-Ehrlich-Institut in Abstimmung mit dem Robert Koch-Institut (RKI) festgelegten Mindestkriterien für Antigen-Tests erfüllen.
more
South Africa has recorded its first case of monkeypox today, 23 June 2022. The Minister of
Health Dr. Joe Phaahla explained that he received a report from the National Health
Laboratory Services’ CEO that they have confirmed through laboratory tests
...
the first case of
monkeypox in South Africa. South Africa's monkeypox patient zero is a 30-year-old man from
Johannesburg.
The South African Health Products Regulatory Authority (SAHPRA) has prepared an
information sheet to better understand monkeypox, the symptoms to treatment.
more
Revised COVID-19 Testing Strategy
recommended
Second edition. June 2022. This revised guidance recommends that access to COVID-19 testing is decentralized as far as possible and made available at health facilities, and through the use of self tests to enable access to care and the mitigation of
...
transmission. Testing should be prioritized for high-risk and vulnerable individuals presenting with acute onset of respiratory illness so that those found to be infected can benefit from clinical care and access to COVID-19 therapeutics and vaccines
more
To meet the need for laboratory diagnosis to be integrated in community care, the new 2022 Update describes point-of-care tests that can be performed both in district laboratories and peripheral healthcare facilities by non-specialist staff. Also in
...
cluded are recently developed self-tests.
more
Les notes d’orientation décrivent les mesures essentielles que les décideurs nationaux et infranationaux peuvent mettre en place concernant les aspects suivants de la lutte contre la COVID-19 : les tests de diagnostic, la prise en charge cliniqu
...
e, la réalisation des cibles en matière de vaccination, le maintien des mesures de lutte anti-infectieuse liées à la maladie dans les établissements de soins de santé, les efforts
visant à instaurer la confiance par la communication sur les risques et la participation communautaire ainsi que la gestion de l’infodémie liée à la COVID-19.
more
Les notes d’orientation décrivent les mesures essentielles que les décideurs aux niveaux national et infranational peuvent mettre en place concernant les aspects suivants : les tests de diagnostic de la COVID19, la prise en charge clinique de l
...
a COVID-19, la réalisation des cibles en matière de vaccination contre la
COVID-19, le maintien des mesures de lutte anti-infectieuse liées à la COVID-19 dans les établissements de soins de santé, les efforts visant à instaurer la confiance par la communication sur les risques et la participation communautaire ainsi que la gestion de l’infodémie liée à la COVID-19. La présente note d’orientation est axée sur la communication sur les risques et la participation communautaire dans le
contexte de la COVID-19.
more
The meeting was held from 26 to 27 March 2018 to review and discuss the following topics:
Advances and challenges in the use of fTLC, and new approaches to detecting mycolactone using monoclonal antibodies (mAbs).
The status of development of rapid diagnostic
...
tests (RDTs) targeting the MUL_3720 protein.
The role of PCR as a reference test, and hurdles in providing a confirmatory diagnosis and in establishing a quality assurance programme.
New molecular tools with potential for implementation at a level lower than in the national or regional reference laboratory, such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA).
The need to harmonize and standardize methods for collection and preparation of specimens, so samples can be referred for diagnosis and stored for evaluation of new diagnostic tests in optimal conditions.
Barriers to accessing early diagnosis and treatment, including coordination at the programme level, and lack of adequate diagnostic tools.
Defining target product profiles (TPPs) to guide the development of new diagnostic tools that can be applied at different levels of the health system. Participants agreed that two TPPs would be developed to address the current gaps: (i) a rapid test for BU diagnosis at the primary health-care level; and (ii) a test for diagnosis of BU that can also assist in treatment monitoring and differential diagnosis at the district hospital or reference centre.
more
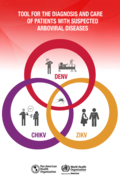
It is Zika virus (ZIKV) that most often causes these neurological effects it appears to be the only arbovirus than can cause congenital malformations such as microcephaly. In any case, more scientific tests are needed to establish the causal relatio
...
nship between the virus and this malformation (7-10).
This document is a practical tool designed to help health workers improve clinical diagnosis and provide timely care for patients infected
with the dengue, chikungunya, or Zika virus. It is intended mainly for
health workers in primary care facilities where laboratory diagnosis of
arboviruses is not always available. However, this guide may also be
very useful in hospitals that provide second- and third-level care, as it
describes the clinical manifestations of each of the three most important
arboviral diseases currently found in the Region, the elements for
differential diagnosis, and their clinical behavior.
more
Diagnostic performance, cost-effectiveness, ease of performance, rapidity and in-field applicability of tests for Soil-transmitted helminth infections.
Self-care interventions are evidence-based, quality drugs, devices, diagnostics and/or digital products which can be provided fully or partially outside of formal health services and can be used with or without the direct supervision of health care personnel.
Where HPV
...
tests are available as part of the national programme, HPV self-sampling offers an additional option to improve cervical cancer screening coverage.
Self-sampling can help reach a global target of 70% coverage of screening by 2030. Women may feel more comfortable taking their own samples, rather than going to see a health worker for cervical cancer screening.
more
The updates are based on evidence emerging from the: Ongoing monitoring of the disease. Protection that the population already developed against COVID-19 through previous infection or vaccination. Epidemiological situation, availability of diagnostic tests
...
and access to therapeutic options.
more
Asthma has a significant impact on people of all ages, particularly children. A lack of universally accepted case definition and confirmatory tests and a poor understanding of major risks interfere with a global response. We aimed to provide global
...
estimates of asthma prevalence and cases in 2019 across four main epidemiological case definitions – current wheezing, ever wheezing, current asthma, and ever asthma. We further investigated major associated factors to determine regional and national distributions of prevalence and cases for current wheezing and ever asthma.
more
The TACT training manuals and patient leaflet were developed as part of the ACT Consortium's initiative to improve malaria case management by promoting the correct use of rapid diagnostic tests (RDTs). The TACT ("Targeting ACTs") initiative aims to
...
ensure that artemisinin-based combination therapies (ACTs) are used appropriately, treating only confirmed malaria cases and guiding alternative care for other febrile illnesses.
Please download the manuals and leaflets from the website
more
Les manuels de formation TACT et la brochure destinée aux patients ont été élaborés dans le cadre de l'initiative du consortium ACT visant à améliorer la prise en charge du paludisme en encourageant l'utilisation correcte des tests de diagnos
...
tic rapide (TDR). L'initiative TACT (Targeting ACTs) vise à garantir l'utilisation appropriée des combinaisons thérapeutiques à base d'artémisinine (ACT), en ne traitant que les cas de paludisme confirmés et en orientant les patients vers d'autres traitements pour les maladies fébriles non liées au paludisme. Les manuels proposent une formation interactive sur le lieu de travail pour aider les personnels de santé à passer d'un traitement présomptif à un diagnostic fondé sur des tests, conformément à la politique de l'OMS. La brochure d'accompagnement destinée aux patients, disponible en anglais et en swahili, s'appuie sur des récits illustrés pour expliquer l'utilité et le fonctionnement des TDR, répondre aux inquiétudes les plus courantes et encourager la confiance dans les résultats des tests. Ces documents, dont la clarté et la compréhension ont été testées au préalable, permettent d'opérer des changements durables dans la pratique clinique et la communication avec les patients au sein des établissements de soins primaires tanzaniens.
Accessed on 03/07/2025.
more
HIV rapid diagnostic test market landscape
recommended
The analysis includes the three most commonly used HIV rapid diagnostic test
(RDT) categories: HIV-only professional use RDTs, dual HIV/syphilis professional use
RDTs, and HIV self-tests (HIVST).