Filter
4490
Text search:
Pregnancy
Featured
Recommendations
382
New Publications
1408
Language
Document type
No document type
2827
Guidelines
591
Studies & Reports
511
Manuals
158
Fact sheets
109
Strategic & Response Plan
106
Training Material
82
Infographics
33
Situation Updates
24
Brochures
20
Resource Platforms
15
Videos
9
Online Courses
3
App
2
Countries / Regions
India
260
Kenya
160
Global
149
Ethiopia
125
Nepal
122
South Africa
115
Uganda
105
Sierra Leone
104
Nigeria
97
Tanzania
95
Zambia
94
Malawi
90
Rwanda
84
Liberia
79
Bangladesh
79
Myanmar / Burma
60
Namibia
57
Ghana
56
Philippines
52
Latin America and the Carribbean
48
Western and Central Europe
44
Lesotho
41
Zimbabwe
39
Cambodia
37
Indonesia
36
Africa
36
Senegal
31
Congo, Democratic Republic of
29
Ukraine
29
Haiti
28
Germany
28
Syria
27
Brazil
27
Botswana
25
Russia
24
South–East Asia Region
22
Mozambique
21
Burkina Faso
20
Asia
20
South Sudan
19
Eswatini/ Swaziland
18
Guinea
15
Cameroon
14
Eastern Europe
14
Venezuela
13
East and Southern Africa
12
Colombia
11
Pakistan
10
Afghanistan
10
Benin
10
Yemen
10
Middle East and North Africa
10
Eastern Europe and Central Asia
9
Thailand
7
West and Central Africa
7
Paraguay
7
Mali
6
USA
6
Somalia
6
Western Pacific Region
6
Côte d’Ivoire / Ivory Coast
5
Lebanon
5
Argentina
5
Madagascar
5
Albania
5
Laos
5
Vietnam
5
North America
5
Sudan
4
Burundi
4
Bolivia
4
Sri Lanka
4
Southern Africa
4
Georgia
4
Togo
3
Turkey
3
Papua New Guinea
3
Central African Republic
3
Peru
3
Tajikistan
3
Iraq
2
Jordan
2
Chad
2
Gambia
2
China
2
North Macedonia
2
Switzerland
2
Honduras
2
Ecuador
2
Guatemala
2
Bhutan
2
Palestine
2
Moldova
2
Egypt
1
Ireland
1
Dominican Republic
1
El Salvador
1
Greece
1
Libya
1
Angola
1
Chile
1
Other region
1
Fiji
1
Solomon Islands
1
Armenia
1
Mongolia
1
Congo-Brazzaville
1
Timor Leste/ East Timor
1
Bulgaria
1
Estonia
1
Lithuania
1
Kyrgyzstan
1
Jamaica
1
France
1
United Kingdom
1
Spain
1
Belarus
1
Latvia
1
Oman
1
United Arab Emirates
1
Authors & Publishers
Publication Years
Category
Countries
2090
Women & Child Health
436
Clinical Guidelines
414
Public Health
175
Capacity Building
127
Key Resources
119
Pharmacy & Technologies
18
Toolboxes
HIV
459
COVID-19
248
Mental Health
226
TB
122
Zika
121
Caregiver
117
Refugee
110
Disability
99
NTDs
91
Malaria
81
NCDs
75
Ebola & Marburg
72
Conflict
68
Pharmacy
59
Planetary Health
52
Rapid Response
44
Global Health Education
43
AMR
25
Natural Hazards
21
Health Financing Toolbox
19
Specific Hazards
11
Cholera
10
2.0 Rapid Response
9
Polio
7
Social Ethics
6
South Sudan
1
Typhoon
1
The World Health Organization (WHO) recommends the use of insecticide-treated nets (ITNs) and intermittent preventive treatment in pregnancy (IPTp) as a cost-effective intervention for the prevention of malaria during
...
Regulation of the Minister of Health of the Republic of Indonesia Number 97 of 2014 on Pre-Pregnancy Health Services, Pregnancy, Labor and Postpartum, Contraception Service, and Sexual Health Servic
...
Regulation of the minister of health of the Republic of Indonesia number 97 of 2014 on pre-pregnancy health services, pregnancy, childbirth, and postpartum, contraceptive service and sexual health s
...
Background: In 2015, 5.3 million babies died in the third trimester of pregnancy and first month following birth. Progress in reducing neonatal mortality and stillbirth rates has lagged behind the substantial progress in reducing postneonatal and ma
...
Every day in 2020, approximately 800 women died from preventable causes related to pregnancy and childbirth - meaning that a woman dies around every two minutes.
Sustainable Development Goal (SDG) target 3.1 is to reduce maternal mortality to less
...
Every year, an estimated 15 million babies are born preterm – before 37 weeks of pregnancy. That is more than 1 in 10 live births. Approximately 1 million children die each year worldwide due to complications from their early birth. Those that sur
...
Accessed June 2014
dapted by PATH from: World Health Organization (WHO)/Department of Reproductive Health and Research.
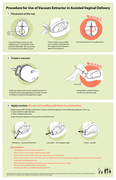
Managing Complications in Pregnancy and Childbirth: a Guide for Midwives and Doctors.
Geneva: WHO; 2007. Available at: http:
...
Infertility is a disease of the male or female reproductive system defined by the failure to achieve a pregnancy after 12 months or more of regular unprotected sexual intercourse. Understanding the magnitude of infertility is critical for developing
...
This statement presents the 2018 definition of skilled health personnel providing care during childbirth (also widely known as a “skilled birth attendants” or SBAs). It results from the recent review and revision of the 2004 joint statement by WHO, FIGO and ICM – Making
...
Chemoprevention is the use of medicines, either alone or in combination, to prevent malaria infection and its consequences. This publication provides standardized approaches for monitoring and evaluating the efficacy of medicines used for intermittent preventive treatment of malaria in
...
The primary audience for these recommendations includes health professionals who are responsible for developing national and local health-care guidelines and protocols and health workers involved in the provision of care to women and their newborns during
...
Over 6 million people worldwide are infected with Trypanosoma cruzi, the protozoan that causes Chagas disease. Endemic in 21 Latin American countries, the disease can be transmitted by vector insects called triatomines — also known as “kissing bugs” —, foods or beverages contaminated with th
...
Fetal alcohol spectrum disorders (FASD) represent a range of physical, mental, and behavioral disabilities caused by alcohol use during pregnancy, or prenatal alcohol exposure (PAE). FASDs are considered to be one of the leading preventable causes o
...
The primary audience for these recommendations includes health professionals who are responsible for developing national and local health-care guidelines and protocols and health workers involved in the provision of care to women and their newborns during
...
Guideline
Iron deficiency is one of the most common forms of nutritional deficiencies, particularly among vulnerable groups such as women, children and low-income populations. Iron deficiency often precedes anaemia, and anaemia during pregnancy ...
Iron deficiency is one of the most common forms of nutritional deficiencies, particularly among vulnerable groups such as women, children and low-income populations. Iron deficiency often precedes anaemia, and anaemia during pregnancy ...
Zika Communication Network (ZCN)
recommended
A reliable one-stop shop for Zika prevention and preparedness materials. ZCN curates essential, evidence-based tools and resources to help health and development professionals minimize the spread of Zika and related negative pregnancy outcomes
There has been important progress for the rights of adolescent girls and women in recent decades, yet millions still struggle to
access the nutritious diets, essential nutrition services and nutrition and care practices they need to prevent malnutrition.
Undernutrition, micronutrient deficiencies
...
Nice multilingual information brochure for refugee women arriving in Germany/Bremen. Includes Information on:
- Learn German
- Education & Employment
- Healthcare & Pregnancy
- Protection against Violence
- Asylum for Women
- Conversation and
...
The primary audience for these recommendations includes health professionals who are responsible for developing national and local health-care guidelines and protocols and health workers involved in the provision of care to women and their newborns during
...
Policy Brief.
WHO recommends that pregnant women receive testing for HIV, syphilis and hepatitis B (HBSAg) at least once during pregnancy, preferably in the first trimester.
Dual HIV/syphilis rapid diagnostic tests (RDTs) can be used as the first
...