Filter
676
Text search:
Pan,
X.
Featured
90
134
Language
Document type
251
160
142
56
23
21
11
4
3
3
1
Countries / Regions
63
56
31
30
16
15
15
12
11
11
9
9
8
8
7
6
6
6
5
5
5
5
5
5
5
5
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
274
71
50
44
25
18
9
Toolboxes
148
40
38
34
27
26
16
15
15
15
15
15
14
13
13
10
8
7
6
6
5
3
3
1
1
The Best Buys for Disease Elimination is a practical, evidence-based guide to the most effective actions for countries to implement in order to eliminate communicable diseases. The guide highlights the efficient use of resources and prioritizing populations in vulnerable situations.
“The Region
...
Para a elaboração deste documento, o Grupo de Farmacovigilância da Rede Pan-americana para a Harmonização Farmacêutica (PARF) baseou-se na perspectiva da OPAS/OMS, a qual considera a Farmacovigilância como componente essencial dos programas d
...
Investigación original / Original research
Rev Panam Salud Publica 35(1), 2014
The article "A 20-year follow-up study on chronic respiratory effects of exposure to cotton dust" evaluates the long-term impact of cotton dust exposure on respiratory health. It followed a cohort of Chinese cotton and silk workers from 1981 to 2001, comparing lung function and symptoms. Results sho
...
A Toolkit for Implementation. Module 2: Facilitator’s guide to the orientation workshop on the IFC framework;
Epidemiology, Control, and Financing
This report aims to improve the assessment of mental health needs in the Americas by providing an updated and nuanced picture of: (a) the disability resulting from mental, substance use, and specific neurological disorders, plus self-harm, alone and in combination with premature mortality; (b) the i
...
This summary covers the preventive approach to breast cancer control and includes prophylactic medications, prophylactic surgery and lifestyle modifications for breast cancer prevention. Health professional training and individual risk assessments and counseling are also discussed.
A Toolkit for Implementation. Module 4: Training guide for facilitators of the participatory community assessment in maternal and newborn health
Se espera que el Panorama 2020 ayude a visibilizar los desafíos de los territorios con peores indicadores en términos de alimentación y nutrición, y que sirva para movilizar el compromiso político y la atención pública en los lugares que sufren mayores rezagos respecto a los promedios naciona
...
Post-traumatic stress disorder (PTSD) and anxiety are both prevalent in trauma-related populations. However, comorbidity of these 2 psychiatric disorders has not been investigated in flood survivors. This study aimed to estimate the extent to which PTSD and anxiety co-occur in flood survivors, and i
...
Pan African Medical Journal 2017;27:215. doi: 10.11604/pamj.2017.27.215.12994
The Member States of the Pan American Health Organization/World Health Organization (PAHO/WHO)
that appear in the tables below have used the assessment instrument for mental health systems (WHOAIMS)
(1), as have Anguilla, the British Virgin Island
...
The international community sits at the tipping pointof a post-‐antibiotic era, where common bacterial infections are no longer treatable with the antibiotic armamentarium that exists. In South Africa, t
...
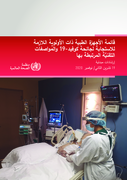
Priority medical devices list for the COVID-19 response and associated technical specifications
recommended
23 February 2021
This document describes the medical devices required for the clinical management of COVID-19, selected and prioritized according to the latest available evidence and interim guidelines. This includes: oxygen therapy, pulse oximeters, patient monitors, thermometers, infusion and suc
...
23 February 2021
This document describes the medical devices required for the clinical management of COVID-19, selected and prioritized according to the latest available evidence and interim guidelines. This includes: oxygen therapy, pulse oximeters, patient monitors, thermometers, infusion and suc
...