Filter
1166
Text search:
adverse
reactions
Featured
150
313
Language
Document type
655
223
116
90
23
21
20
8
4
3
1
1
Countries / Regions
80
36
31
28
27
27
26
23
22
17
17
17
16
16
16
12
11
11
11
10
10
9
8
8
7
7
7
7
6
6
5
4
4
4
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
424
154
61
48
43
19
16
Toolboxes
132
110
80
72
64
50
30
26
26
25
24
20
17
16
15
12
10
8
7
6
6
6
5
1
This Indicator-Based Pharmacovigilance Assessment Tool (IPAT) was developed as a comprehensive performance metric for pharmacovigilance and medicine safety systems.
Accessed November, 2017
Accessed November 2017
Therapeutics Information and Pharmacovigilance Centre | TIPC
Made under Section 5 (c) of the Tanzania Food, Drugs and Cosmetics Act, 2003 | Second Edition
The Zimbabwe National Pharmacovigilance Policy Handbook, 2nd Edition updates the November 2013 version to indicate the Zimbabwe National Pharmacovigilance (PV) Centre’s compliance with the WHO Pharmacovigilance Indicators Handbook 2015.
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
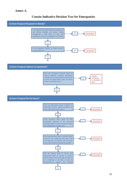
The document contains 2 decision trees, the first short one is to act fast and the second is to design better interventions