Filter
739
Text search:
specimen
transport
Featured
133
232
Language
Document type
396
186
56
49
26
13
7
4
1
1
Countries / Regions
58
30
29
27
26
24
19
18
16
16
13
12
12
11
9
9
9
9
8
8
7
6
6
6
6
5
5
5
5
4
3
3
3
3
3
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
Publication Years
Category
320
93
50
28
24
14
11
Toolboxes
101
100
61
34
31
29
26
17
15
12
9
9
8
8
8
6
4
4
3
2
2
2
Laboratory testing for the monkeypox virus
recommended
Any individual that meets the suspected case definition of monkeypox should be offered testing in appropriately equipped laboratories by staff trained in the relevant technical and safety procedures. Confirmation of monkeypox virus infection is based on nucleic acid amplification testing (NAAT), usi
...
The Practical manual on laboratory strengthening, 2022 update provides practical guidance on implementation of WHO recommendations and best practices for TB laboratory strengthening. It is an updated version of the GLI Practical Guide to Laboratory Strengthening published in 2017 and provides the la
...
Laboratory biosafety guidance related to coronavirus disease (COVID-19): Interim guidance, 28 January 2021
recommended
The latest update (28 January 2021) includes the following addition and revision:
biosafety aspects for working with antigen-detecting rapid diagnostic test;
handling new variants of SARS-CoV-2 in the laboratory;
updated assay decontamination before disposal;
personal protectiv
...
In 2007, WHO warned that infectious diseases are emerging and re-emerging at a rate that has not been seen before. The potential for infectious diseases to spread rapidly results in high morbidity and mortality, causing a potential global public health treat of major concern.
Several factors are
...
The National Guidelines for HIV-1 Viral Load Laboratory Testing support plans to scale up viral load (VL) testing to reach the 90-90-90 targets in India. This phased scale-up includes the setup of 70 additional VL testing laboratories nationally. These guidelines include laboratory design considerat
...
This document provides technical guidance for manufacturers seeking World Health Organization (WHO) prequalification of in vitro diagnostic devices (IVDs) for malaria, with a focus on rapid diagnostic tests (RDTs) for symptomatic patients. It summarises the minimum performance requirements, includin
...
This document provides technical guidance for manufacturers seeking World Health Organization (WHO) prequalification of in vitro diagnostic devices (IVDs) for malaria, with a focus on rapid diagnostic tests (RDTs) for symptomatic patients. It summarises the minimum performance requirements, includin
...
Household transmission investigation protocol for 2019-novel coronavirus (2019-nCoV) infection
recommended
The household transmission investigation is a case-ascertained prospective study of all identified household contacts of a laboratory confirmed 2019-nCoV infection (see 2.2 Study population). It is intended to provide rapid and early information on the clinical, epidemiological and virological chara
...
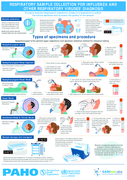
Respiratory sample collection for Influenza and other respiratory viruses diagnosis - Infographic
The main objectives of these guidelines are to:
1. contribute to the quality assurance of medicinal plant materials used as the source for herbal medicines to improve the quality, safety and efficacy of finished herbal products; 2. guide the formulation of national and/or regional GACP guideli ...
1. contribute to the quality assurance of medicinal plant materials used as the source for herbal medicines to improve the quality, safety and efficacy of finished herbal products; 2. guide the formulation of national and/or regional GACP guideli ...
Interim Guidance 31 march 2020
WHO has established a shipment mechanism to expedite and cover the costs of the shipment of clinical samples from patients with suspected COVID-19 from the country of collection to one of the WHO reference laboratories providing confirmatory molecular testing for COVI
...
Improve identification, verification, communication and coordination.
This is a case-ascertained prospective investigation of all identified health care contacts working in a health care facility in which a laboratory confirmed 2019-nCoV infected patient (see 2.2 Study population) receives care. Note that this study can be done in health care facilities at all 3 level
...
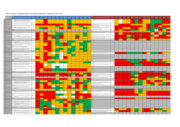
The dashboard is based on assessments made by the International Preparedness Strengthening missions to 14 priority countries against each of the activities outlined in the WHO EVD Checklist at the time of each mission. Updates indicating progress against each of the indicators will be added on an o
...
This job aid provides information for laboratorians about how to receive, process, and store dried blood spot specimens collected for early infant diagnosis, viral load, or drug resistance testing.