Filter
3326
Text search:
drug
safety
Featured
370
913
Language
3151
83
76
63
34
33
24
10
9
8
7
6
5
5
5
5
5
4
4
4
4
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Document type
1791
564
477
204
129
54
54
16
11
9
8
4
1
1
Countries / Regions
177
102
101
87
80
78
73
63
60
56
56
52
50
49
45
44
44
43
35
35
29
26
24
23
22
22
20
19
19
18
17
16
15
15
15
14
13
13
11
11
10
10
10
9
9
8
8
8
8
7
7
7
6
6
6
6
6
6
5
5
4
4
4
4
4
4
3
3
3
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Authors & Publishers
498
184
122
80
76
68
50
42
39
31
28
25
24
23
19
17
17
16
16
15
15
15
14
13
12
12
12
11
11
11
10
10
10
10
10
10
10
10
9
9
9
9
8
8
8
8
8
8
8
8
8
8
7
7
7
7
7
7
7
7
7
7
7
7
7
6
6
6
6
6
6
6
6
6
6
6
6
6
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
5
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
4
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
3
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Publication Years
863
2110
328
22
2
1
Category
1289
313
174
149
129
54
50
Toolboxes
318
246
225
219
210
157
121
86
71
61
58
57
48
45
40
39
28
22
20
20
19
17
15
14
6
EXPERT OPINION ON DRUG SAFETY 2018, VOL. 17, NO. 11, 1129–1144.
Malaria during and after pregnancy contributes significantly to maternal mortality and adverse fetal outcomes. While effective and
...
safe antimalarial treatments are essential, quinine — an older, less effective drug — has long been favoured due to the limited safety data available on newer drugs. This review summarises the results of human studies investigating the safety and efficacy of antimalarial drugs during pregnancy and lactation.
more
In preparing this paper, the Pharmacovigilance Group of the Pan American Health Organization’s Pan American Network for Drug Regulatory Harmonization (PANDRH) adopted the perspective of PAHO/WHO, which considers Pharmacovigilance, an essential com
...
ponent of public health programs. Its intention was to facilitate the development of pharmacovigilance systems in the Region of the Americas and improve, strengthen, and promote the adoption of good practices to improve safety for patients and the general population, based on the needs of the Region.
Document also available in Spanish and Portuguese!
more
The WHO Pharmaceuticals Newsletter provides you with the latest information on the safety of medicinal products and regulatory actions taken by authorities around the world.
In addition, this edition includes summary and recommendations from the vi
...
rtual meeting of the members of the WHO Programme for International Drug Monitoring (PIDM) and other partners, which was held on 20 October 2022.
more
The Pharmacovigilance team in WHO aims to assure the safety of medicines and vaccines by ensuring reliable and timely exchange of information on safety issues, promoting pharmacovigilance activities
...
throughout the Organization and encouraging participation in the WHO Programme for International Drug Monitoring. This text was developed in consultation with the WHO Collaborating Centre for International Drug Monitoring and the national pharmacovigilance centres participating in the WHO Programme for International Drug Monitoring.
more
Ivermectin is an antiparasitic drug approved for the treatment of parasitic infections, including strongyloidiasis and onchocerciasis in humans. There is a reported increase in the use of ivermectin for the prevention and treatment of COVID-19 by th
...
e public in African Union Member States.
Currently, there is:
1. No scientific evidence from pre-clinical studies on the therapeutic effect of ivermectin for the management of COVID-19;
2. No evidence of its clinical efficacy for the management of patients with asymptomatic, mild, moderate or severe COVID-19; and
3. No safety data regarding the use of ivermectin for COVID-19 in the majority of the published studies.
more
Pregnancy often results in exclusion from clinical trials of antiretroviral (ARV) drugs, resulting in limited data on pharmacokinetics (PK), drug safety, and the efficacy of new ARV drugs in pregnan
...
cy and lactation. However, pregnancy, lactation, or the potential for pregnancy should not preclude the use of drug regimens that would be chosen for people who are not pregnant, unless adequate drug levels are not likely to be attained in pregnancy or known adverse effects outweigh potential benefits
more
National Guidelines for the Treatment of Malaria - 2019
South African Malaria Elimination Committee (SAMC)
National Department of Health South Africa
(2019)
CC
These guidelines are based on the 3rd Edition of the WHO Guidelines (Published 2015) World Health Organization’s Guidelines for the treatment of malaria. Additional literature surveys have been undertaken. Factors that were considered in the choice of therapeutic options included effectiveness,
...
safety, and impact on malaria transmission and on the emergence and spread of antimalarial drug resistance. On-going surveillance is critical given the spread of artemisinin resistance in Southeast Asia, although not yet confirmed anywhere in Africa. The guidelines on the treatment of malaria in South Africa aim to facilitate effective, appropriate and timeous treatment of malaria, thereby reducing the burden of this disease in our communities. This is essential to further reduce the malaria case fatality rates currently recorded in South Africa, to decrease malaria transmission and to limit resistance to antimalarial drugs.
more
Antimalarial chemotherapy is crucial for reducing morbidity, mortality, and drug resistance, and is the cornerstone of malaria control. Existing antimalarial drugs act at different stages of the parasite’s life cycle. These drugs range from classi
...
c agents such as chloroquine and quinine to newer artemisinin derivatives. They include tissue schizonticides, blood schizonticides, gametocytocides, and sporontocides. Artemisinin and its derivatives are the most effective and fastest-acting treatment against drug-resistant Plasmodium falciparum, achieving rapid parasite clearance and reducing transmission potential. Other key drugs include mefloquine, halofantrine, proguanil, sulfadoxine–pyrimethamine, atovaquone–proguanil, tetracyclines, clindamycin and azithromycin. Each of these drugs has a specific mechanism of action, pharmacokinetics, efficacy, safety profile and contraindications. Rational drug combinations and adherence to national treatment guidelines are essential for managing resistance, ensuring safety in vulnerable populations such as children and pregnant women, and optimising therapeutic outcomes in cases of both uncomplicated and severe malaria.
more
Clinical guideline, Methods, Evidence and Recommendations
In this guideline the following is covered: information needs of people with chronic hep
titis B and their carers; where children, young people and adults with chronic hepatitis B a-
should be assessed; assessment of liver disease, includi
...
ng the use of non-invasive tests and genotype testing; criteria for offering antiviral treatment; the efficacy, safety and cost effectiveness of currently available treatments; selection of first-line therapy; management of treatment failure or drug resistance; prophylactic treatment during im-
munosuppressive therapy; and monitoring for treatment response
more
This video highlights the significant risks that malaria poses to pregnant and breastfeeding women in malaria-endemic regions. It follows the stories of Angavu from Kenya, Moyinoluwa from Nigeria and Lamai from Thailand. It emphasises the severe consequences that malaria can have during pregnancy, i
...
ncluding miscarriage, stillbirth, low birth weight and maternal death. Due to safety concerns, pregnant women are often excluded from antimalarial drug trials, which causes long delays in effective medicines becoming available for this vulnerable group. To address this issue, the Medicines for Malaria Venture (MMV) launched the Malaria in Mothers and Babies (MiMBa) initiative, which aims to accelerate the discovery, development, and delivery of safe antimalarial treatments for pregnant and breastfeeding women. The initiative aims to close critical gaps in research, drug development, and access, ensuring that these women and their babies are better protected against malaria. The video calls for continued efforts to address the needs of underserved populations most affected by malaria.
more
Combination therapy is a cornerstone of modern malaria treatment, particularly in the context of widespread multidrug resistance. Using two or more antimalarial drugs with different mechanisms simultaneously enhances efficacy, shortens treatment duration, improves compliance and delays the developme
...
nt of resistance. Artemisinin-based combination therapies (ACTs), such as artemether–lumefantrine, artesunate–amodiaquine and artesunate–sulfadoxine/pyrimethamine, are highly effective in rapidly clearing parasites and reducing gametocyte carriage. They are also generally well tolerated. Non-artemisinin combinations, quinine-based regimens and novel combinations (e.g. piperaquine–dihydroartemisinin) offer alternative therapeutic options, although clinical experience with these remains limited. Although ACTs are the preferred first-line treatment, factors such as cost, local drug resistance patterns, safety during pregnancy and paediatric use must inform implementation and policy decisions.
more
Dengue is the fastest spreading, mosquito-borne viral infectious disease worldwide, with remarkable morbidity and mortality. In the past decades, profound contributions have been made towards understanding its epidemiology, including disease burden and distributions, risk factors, and control and pr
...
evention practices. Dengue continues to disseminate to new areas, including high latitude regions, and a new serotype (dengue virus serotype 5) has been identified. Vaccine research has made new progress, in which the licensed yellow fever and dengue virus quadrivalent chimeric vaccine is now under further safety assessment. In disease surveillance, because of its operational simplicity, rapidity, capability, and utility as an indicator of disease severity, dengue virus NS1 antigen detection has great promotion and application value among primary health care institutions. Vector control progress has driven new breakthroughs in biotechnology, including Wolbachia-infected Aedes and genetically modified Aedes. Both Aedes variants have been used to block transmission of the dengue virus through population replacement and suppression. In the future, vector control should still be pursued as a key measure to prevent transmission, along with anti-viral drug and vaccine research.
more
This course is based on the Manual on Safety in Administering Medicines for Neglected Tropical Diseases which provides practical tools, training modules and jobs aids to further improve the planning, preparation, and monitoring of safe administratio
...
n of NTD medicines.
more
Road safety is an issue that does not receive anywhere near the attention it deserves – and it really is one
of our great opportunities to save lives around the world
COVID-19 Vaccines: 1 Safety Surveillance 2 Manual
While there is no indication that pregnant women have an increased susceptibility to infection with SARS-CoV-2, there is evidence that pregnancy may increase the risk of severe illness and mortality
...
from COVID-19 disease in comparison with non-pregnant women of reproductive age. As seen with non-pregnant women, a high proportion of pregnant women have asymptomatic SARS-CoV-2 infection and severe disease is associated with recognized medical (e.g., high body-mass index (BMI), diabetes, pre-existing pulmonary or cardiac conditions) and social (e.g., social deprivation, ethnicity) risk factors. Pregnant women with symptomatic COVID-19 appear to have an increased risk of intensive care unit admission, mechanical ventilation and death in comparison with non-pregnant women of reproductive age, although the absolute risks remain low. COVID-19 may increase the risk of preterm birth, compared with pregnant women without COVID-19, although the evidence is inconclusive.
more
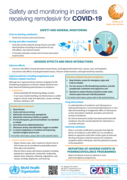
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
This series of supportive tools are based on the WHO Therapeutics and COVID-19: living guideline. They are intended to provide supportive information for healthcare workers who are prescribing, administering and monitoring patients receiving nirmatrelvir-ritonavir for non-severe COVID-19.
Pharmaceutical News
Evaluation of Saccharide Content of the WHO 2nd International Standard for Haemophilus Influenzae Polysaccharide Polyribosyl Ribitol Phosphate (PRP) by HPAECPAD Analysis Following Acid Hydrolysis
Consultation Documents
Lamivudine and tenofovir disoproxil fumarate tablets (lami
...
vudini et tenofoviri disoproxili fumarati compressi)
Tenofovir disoproxil fumarate tablets (tenofoviri disoproxili fumarati compressi)
ATC/DDD Classification
Temporary
Final
more