Filter
6054
Text search:
diagnostic
Featured
Recommendations
627
New Publications
1420
Language
Document type
No document type
3153
Guidelines
1002
Studies & Reports
909
Manuals
322
Strategic & Response Plan
264
Fact sheets
164
Training Material
104
Situation Updates
41
Resource Platforms
31
Infographics
28
Brochures
14
Online Courses
12
Videos
7
App
2
Countries / Regions
India
264
Global
251
Senegal
144
Congo, Democratic Republic of
139
Kenya
127
Western and Central Europe
118
Africa
118
South Africa
107
Nigeria
95
Latin America and the Carribbean
91
Ethiopia
89
Burkina Faso
87
Sierra Leone
85
Uganda
84
Tanzania
84
Liberia
83
Rwanda
81
Zambia
65
Ghana
63
Nepal
62
Benin
59
Eastern Europe
59
Malawi
58
Guinea
54
Bangladesh
49
Namibia
49
Haiti
48
Ukraine
46
West and Central Africa
43
Russia
43
Cameroon
40
Madagascar
38
Myanmar / Burma
38
Philippines
33
Brazil
33
Indonesia
31
East and Southern Africa
30
Asia
30
Mali
29
South–East Asia Region
29
Zimbabwe
28
Syria
28
Middle East and North Africa
27
Cambodia
26
Germany
25
Mozambique
24
Eastern Europe and Central Asia
23
Central African Republic
22
Côte d’Ivoire / Ivory Coast
21
Botswana
17
Lesotho
17
Colombia
16
Argentina
15
Pakistan
14
Peru
14
Eswatini/ Swaziland
14
Venezuela
14
Niger
13
Togo
13
Chad
13
El Salvador
12
South Sudan
10
North America
10
Afghanistan
9
Lebanon
9
Yemen
9
USA
8
Ecuador
8
Angola
8
Georgia
8
Sudan
7
Western Pacific Region
7
Paraguay
7
France
7
Iraq
6
Jordan
6
China
6
Albania
6
Tajikistan
6
Morocco
5
Thailand
5
Burundi
5
Sri Lanka
5
Chile
5
Armenia
5
Saudi Arabia
4
North Macedonia
4
Bolivia
4
Laos
4
Canada
4
Estonia
4
Iran
4
Vietnam
4
Somalia
3
Egypt
3
Dominican Republic
3
Libya
3
Guatemala
3
Luxembourg
3
Bhutan
3
Palestine
3
Congo-Brazzaville
3
Timor Leste/ East Timor
3
Romania
3
Moldova
3
Spain
3
Turkey
2
Gambia
2
Mauritania
2
Singapore
2
Papua New Guinea
2
Hungary
2
Honduras
2
Nicaragua
2
Other region
2
Poland
2
Djibouti
2
Mongolia
2
Southern Africa
2
Qatar
2
Bulgaria
2
Lithuania
2
Gabon
2
Belgium
2
Tunisia
2
United Kingdom
2
Guyana
2
Ireland
1
Cuba
1
Greece
1
Mexico
1
Fiji
1
Slovakia
1
Australia
1
Kazakhstan
1
Uruguay
1
Turkmenistan
1
Japan
1
Denmark
1
Maldives
1
Portugal
1
Belarus
1
Belize
1
Costa Rica
1
Panama
1
Authors & Publishers
Publication Years
Category
Countries
2243
Clinical Guidelines
595
Public Health
281
Women & Child Health
181
Key Resources
166
Pharmacy & Technologies
132
Capacity Building
92
Annual Report MEDBOX
2
Toolboxes
TB
576
Mental Health
528
COVID-19
501
HIV
412
NTDs
303
AMR
243
Ebola & Marburg
218
Rapid Response
188
Malaria
173
Caregiver
149
NCDs
126
Pharmacy
101
Disability
99
Planetary Health
82
2.0 Rapid Response
74
Conflict
68
Zika
67
Refugee
66
Cholera
63
Health Financing Toolbox
47
Global Health Education
39
Polio
30
Natural Hazards
27
Specific Hazards
21
Social Ethics
3
South Sudan
1
Africa is experiencing an increasing burden of cardiac arrhythmias. Unfortunately, the expanding need for appropriate care remains largely unmet because of inadequate funding, shortage of essential medical expertise, and the high cost of diagnostic
...
Laboratory diagnosis of Buruli ulcer
recommended
A manual for health care providers.
This manual provides expert guidance on the laboratory techniques and procedures used in the diagnosis of Buruli ulcer, a disease caused by Mycobacterium ulcerans. Aimed at laboratory technicians and scientists working on this disease, the manual details the exac
...
La récente augmentation du nombre de cas de microcéphalie et d’autres troubles neurologiques potentiellement associés à une infection à virus Zika a engendré une recrudescence des demandes de dépistage en laboratoire de cette infection. Les groupes prioritaires pour un test de
...
Peripheral artery disease (PAD) is the most underdiagnosed, underestimated and undertreated of the atherosclerotic vascular diseases despite its poor prognosis. There may be racial or contextual differences in the Asia-Pacific region as to epidemiology, availability of
...
The South African Heart Association's webpage provides comprehensive information about rheumatic heart disease (RHD). It addresses the causes, symptoms, and diagnostic methods of this condition. Additionally, it outlines prevention strategies and tr
...
Au cours de la dernière décennie, les données de séquençage génétique des agents pathogènes ont gagné en importance et contribuent désormais de façon déterminante à la détection et à la maîtrise des flambées de maladies infectieuses, en facilitant la mise au point de
...
This policy guideIine is intended for use by healthcare professionals invoIved in the management of Covid-19 in South Africa. The document provides guidance on diagnostic tests available, on antigen test performance and accessibility. 1t focuses on
...
Interim Recommendation published 19 September 2014. These recommendations reflect current understanding of Ebola virus disease (EVD) and are intended for national laboratory staff performing diagnostic testing to detect Ebola virus.
Barriers to the prompt and effective diagnosis and treatment of malaria exist at both the community and health facility level. Household surveys measure malaria case management at the population level with standard indicators that assess treatment-seeking behavior, access to
...
The article provides a comprehensive overview of chronic obstructive pulmonary disease (COPD), covering its causes, symptoms, diagnostic methods, classification of severity, treatment options, and management strategies, with a focus on risk factors,
...
Diagnosis and Treatment Outcomes of Tuberculosis in Relation to Gender and HIV Status in South Benin
Journal of Tuberculosis Research, 2017, 5, 189-200
Background: In Benin, little is known about the influence of both gender and
HIV-status on diagnostic patterns and treatment outcomes of Tuberculosis
(TB) patients. Objective: To assess whether
...
Given the current situation of the COVID-19 pandemic, countries are advised to continue adopting the TB diagnostic algorithms recommended by PAHO / WHO.
Despite the differences in the modes of transmission of TB and COVID-19, certain personal prote
...
Zika and dengue viruses remain significant public health threats. These viruses share the same Aedes (Stegomyia) mosquito vectors and geographic distributions but infections cannot be readily distinguished clinically and need to be differentiated from each other, and from other circulating arboviral
...
L’Organisation mondiale de la Santé (OMS) recommande aux services de dépistage du VIH (SDV) de remplacer les Western blots et les immunodosages sur bandelettes par des tests plus simples de dépistage du VIH, comme des tests de diagnostic rapide
...
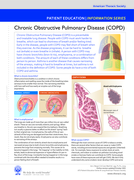
The document by the American Thoracic Society provides an overview of Chronic Obstructive Pulmonary Disease (COPD), explaining its causes, such as smoking and environmental factors, symptoms like breathlessness and chronic cough, and diagnostic meth
...
Cardiovascular diseases remain the leading cause of morbidity and mortality worldwide. There are significant differences in the burden of cardiovascular disease and associated risk factors, across high-income countries and low- and middle-income countries. Cardiac imaging by echocardiography, cardia
...
Le document fournit des directives complètes pour la gestion des épidémies de choléra, y compris la prévention, le diagnostic et le traitement. Il met l'accent sur les mesures de réponse rapide, l'importance de la thérapie de réhydratation (
...
Globally each year, millions of people suffer illness or lose their lives because the vaccines, medicines and diagnostic tests that they need are either unavailable or unaffordable – and this lack of access to medicine is acute in low- and middle-
...
Accessed on 10.03.2020
L’Enquête sur les Indicateurs du Paludisme, effectuée au Burkina Faso (EIPBF) en 2017-2018, fournit des données sur les indicateurs du paludisme tels que la prévention chez les femmes enceintes et les enfants, le diagnostic
...
ScientificWorldJournal. 2007 Nov 12;7:1799-809.
Research indicates that family reaction to the birth of a disabled child changes according to the type of disability and the child's diagnostic category. The differences are probably an indirect conse
...