Filter
5093
Filtered Results: 5093
Text search:
medcines
Featured
Recommendations
512
New Publications
1396
Language
Document type
No document type
2559
Studies & Reports
860
Guidelines
735
Manuals
293
Strategic & Response Plan
230
Fact sheets
150
Training Material
94
Situation Updates
68
Brochures
31
Resource Platforms
31
Infographics
26
Online Courses
10
Videos
6
Countries / Regions
India
249
Global
226
Kenya
155
South Africa
116
Uganda
112
Ethiopia
108
Nepal
96
Sierra Leone
95
Africa
94
Nigeria
89
Liberia
89
Western and Central Europe
83
Bangladesh
78
Tanzania
76
Malawi
75
Latin America and the Carribbean
74
Zambia
74
Myanmar / Burma
74
Ukraine
73
Ghana
72
Namibia
61
Rwanda
58
Syria
56
Congo, Democratic Republic of
46
Philippines
42
Venezuela
41
Zimbabwe
40
South–East Asia Region
37
Eastern Europe
37
Mozambique
32
Haiti
32
Asia
31
West and Central Africa
27
Indonesia
27
Guinea
26
Burkina Faso
25
Cambodia
24
East and Southern Africa
24
Yemen
24
Lesotho
23
Senegal
23
Middle East and North Africa
23
Russia
23
Botswana
22
South Sudan
22
Brazil
19
Germany
18
Eastern Europe and Central Asia
17
Madagascar
16
Eswatini/ Swaziland
16
Cameroon
15
Pakistan
15
Sudan
13
Benin
12
Afghanistan
12
Western Pacific Region
12
North America
12
Tajikistan
10
Colombia
10
Central African Republic
10
Mali
10
Somalia
10
Thailand
9
Paraguay
9
Lebanon
9
Moldova
9
Vietnam
8
Georgia
8
Peru
7
North Macedonia
6
Laos
6
Jordan
6
Côte d’Ivoire / Ivory Coast
6
Bolivia
6
El Salvador
5
China
5
Albania
5
Kyrgyzstan
5
Argentina
5
Sri Lanka
5
Palestine
5
Poland
5
Iraq
5
Mexico
4
Turkey
4
Iran
4
Niger
4
Hungary
4
United Kingdom
4
Southern Africa
4
Libya
4
Bhutan
4
Ecuador
4
Timor Leste/ East Timor
3
Serbia
3
Kazakhstan
3
Fiji
3
Morocco
3
Armenia
3
USA
3
Saudi Arabia
3
Jamaica
3
Mauritius
3
Papua New Guinea
3
Egypt
3
Romania
3
Angola
3
Chad
3
Australia
2
Gambia
2
Chile
2
Canada
2
Uzbekistan
2
Qatar
2
Bulgaria
2
Guinea-Bissau
2
Gabon
2
Turkmenistan
2
Japan
2
Tunisia
2
Belarus
2
Nicaragua
2
Honduras
2
Guatemala
2
Switzerland
1
Togo
1
Congo-Brazzaville
1
Burundi
1
Malaysia
1
Mongolia
1
Dominican Republic
1
Lithuania
1
Uruguay
1
Denmark
1
France
1
Maldives
1
Norway
1
Portugal
1
Spain
1
Israel
1
Latvia
1
Belize
1
Costa Rica
1
Oman
1
Panama
1
Djibouti
1
Slovakia
1
Greece
1
Solomon Islands
1
Authors & Publishers
Publication Years
Category
Countries
2043
Clinical Guidelines
437
Public Health
293
Women & Child Health
206
Key Resources
180
Capacity Building
88
Pharmacy & Technologies
60
Annual Report MEDBOX
2
Toolboxes
HIV
390
COVID-19
385
TB
304
AMR
296
Mental Health
288
Pharmacy
253
NCDs
157
NTDs
147
Caregiver
132
Conflict
131
Ebola & Marburg
113
Disability
107
Malaria
103
Refugee
93
Planetary Health
92
Global Health Education
79
Rapid Response
68
Health Financing Toolbox
63
Natural Hazards
52
2.0 Rapid Response
27
Zika
21
Cholera
19
Polio
12
Specific Hazards
11
Social Ethics
7
Typhoon
1
This Tuberculosis guide has been developed jointly by Médecins Sans Frontières and Partners In Health. It aims at providing useful information to the clinicians and health staff for the comprehensive management of tuberculosis. Forms of susceptible and resistant tuberculosis, tuberculosis in child...
The Zimbabwe National Pharmacovigilance Policy Handbook, 2nd Edition updates the November 2013 version to indicate the Zimbabwe National Pharmacovigilance (PV) Centre’s compliance with the WHO Pharmacovigilance Indicators Handbook 2015.
Accessed: 08.10.2019
Based on the National Guidelines for the Management of Tuberculosis in Children 2013, Department of Health, South Africa.
A review of prospects for existing antibiotics ad new therapeutics
Chapter in Clinical guidelines -Diagnosis and Treatment manual
Le flyer „MMV Strategy“ présente la stratégie de la Medicines for Malaria Venture (MMV) pour lutter contre le paludisme. Il met en avant les objectifs clés de l'organisation, qui se concentre sur la recherche et le développement de nouveaux médicaments antipaludiques. Le document décrit le...
The webpage from Medicines for Malaria Venture (MMV) focuses on efforts to develop and provide child-friendly antimalarial treatments. It highlights the challenges of treating malaria in children, who are among the most vulnerable to the disease, and the need for safe, effective, and easy-to-adminis...
Step Up for TB 2020 Tuberculosis Policies in 37 Countries A survey of prevention, testing, and treatment policies and practices.
The country scorecard reflects how many of 14 internationally recommended key policies are in place at the national level, based on the Step Up for TB 2020 report survey...
This comprehensive reference document is intended for those responsible for training in SMC (Seasonal Malaria Chemoprevention). It assumes that SMC will be implemented using the Community Health Worker strategy.
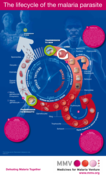
This document by Medicines for Malaria Venture (MMV) highlights the significant burden of malaria on children worldwide, emphasizing the need for effective prevention, diagnosis, and treatment strategies. It reviews current challenges and progress in combating pediatric malaria, advocating for conti...
Dans certaines parties d’Afrique, la transmission du paludisme a lieu principalement pendant les trois ou quatre mois de la saison des pluies. Environ 39 millions d’enfants de moins de cinq ans vivent dans les zones de transmission saisonnière, où on dénombre environ 34 millions de cas et où...
Where malaria transmission is seasonal, notably in Africa’s Sahel region, children of all ages most at risk of severe malaria are protected through SMC. This intervention consists of full antimalarial treatment courses of sulfadoxine-pyrimethamine and amodiaquine (SPAQ), administered monthly (28 d...
The safety of medicines in Zambia - why health workers need to take action | Produced by the National Pharmacovigilance Unit (NPVU)
First edition, November 1997 | Revised July 2002
An overview of COVID-19 Vaccine AstraZeneca and why it is authorised in the EU. Available in 22 languages
18 Febr. 2021
Interim Assessement Report
The EMA review was started by the Agency’s Committee for Medicinal Products for Human Use (CHMP) to support decision-making by health authorities. This first interim report includes information on seven experimental medicines intended for the treatment of people infecte...