Filter
892
Filtered Results: 892
Text search:
Hemorrhagic
fevers
Featured
Recommendations
138
New Publications
276
Language
Document type
No document type
530
Guidelines
163
Studies & Reports
65
Manuals
54
Fact sheets
20
Training Material
16
Strategic & Response Plan
15
Situation Updates
10
Infographics
8
Online Courses
5
Brochures
2
Resource Platforms
2
Videos
2
Countries / Regions
Liberia
92
Sierra Leone
74
Guinea
50
Congo, Democratic Republic of
46
Nigeria
43
Rwanda
38
India
37
Uganda
30
Senegal
28
Burkina Faso
28
Global
26
Côte d’Ivoire / Ivory Coast
25
Mali
25
Ethiopia
20
Ghana
18
South Africa
15
Kenya
14
Philippines
13
West and Central Africa
13
Africa
12
Niger
10
Guinea-Bissau
10
Bangladesh
10
Chad
9
Western and Central Europe
8
Indonesia
8
Myanmar / Burma
8
Nepal
7
East and Southern Africa
7
Germany
6
South Sudan
6
Tanzania
5
Malawi
4
Haiti
4
Cambodia
4
South–East Asia Region
4
Brazil
4
Namibia
4
Sudan
4
Ukraine
3
Lesotho
3
Togo
3
Cameroon
3
Afghanistan
3
Zimbabwe
3
Paraguay
3
Thailand
2
Burundi
2
Mozambique
2
Asia
2
Latin America and the Carribbean
2
Middle East and North Africa
2
Central African Republic
2
Angola
2
Zambia
2
Eswatini/ Swaziland
2
North Macedonia
1
Colombia
1
Benin
1
Gambia
1
Chile
1
Syria
1
Mauritania
1
USA
1
Dominican Republic
1
Venezuela
1
Saudi Arabia
1
Bolivia
1
Russia
1
Yemen
1
Botswana
1
Somalia
1
Madagascar
1
Pakistan
1
Authors & Publishers
Publication Years
Category
Countries
281
Clinical Guidelines
126
Key Resources
63
Public Health
42
Capacity Building
27
Women & Child Health
27
Pharmacy & Technologies
9
Toolboxes
Ebola & Marburg
294
Rapid Response
69
NTDs
51
COVID-19
43
2.0 Rapid Response
35
Caregiver
22
Pharmacy
21
Zika
19
Planetary Health
18
AMR
15
Specific Hazards
13
Mental Health
11
Natural Hazards
11
Conflict
11
Malaria
9
Global Health Education
8
HIV
6
NCDs
6
Refugee
3
Cholera
3
TB
2
Health Financing Toolbox
2
Polio
1
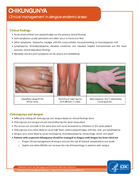
Clinical Management in dengue-endemic areas
Sudan virus disease is a severe, often fatal illness affecting humans and other primates that is due to Orthoebolavirus sudanense (Sudan virus), a viral species belonging to the same genus of the virus causing Ebola virus disease. This webinar will provide an overview of the current outbreak of Suda...
- The goal of diagnostic testing for Ebola and Marburg virus diseases is to identify cases to provide timely and appropriate care and to stop disease transmission.
- All individuals meeting the case definition for Ebola or Marburg virus diseases should be tested.
- The recommended sample type ...
Hand hygiene in health care in the context of Filovirus disease outbreak response. Rapid advice guideline
recommended
This document provides a summary of the recommendations for hand hygiene best practices to be performed by health workers providing care and/or support to patients with filovirus infection (Ebola and Marburg viruses).
Presentation is current through November 21, 2014 and will be updated every Friday by 5pm. For the most up-to-date information, please visit www.cdc.gov/ebola.
*Presentation contains materials from CDC, MSF, and WHO
Interim emergency guidelines
This technical briefing paper details the construction and setup of medical isolation facilities in support of infectious disease outbreak responses.
The African region reports the highest number of health emergencies of all the WHO regions every year: an average of
2-3 new events every week
Risk Communication and Community Engagement (RCCE) is an essential part of any disease outbreak response. Risk communication in the context of an Ebola outbreak refers to real time exchange of information, opinion and advice between frontline responders and people who are faced with the threat of Eb...
The checklist is based on efforts by various national and international institutions, including WHO, CDC and UN OCHA. It
identifies 10 key components and tasks for both countries and the international community. This tool establishes timelines
within which to complete tasks of 30, 60 and 90 days r...
On 4 September 2025, the Ministry of Health of the Democratic Republic of the Congo (DRC) declared an outbreak of Ebola Virus Disease (EVD) in Kasai Province, following confirmation of Zaire ebolavirus by the National Institute of Biomedical Research (INRB) in Bulape and Mweka Health Zones. As of 19...