- Cumming School of Medicine, Departments of Psychiatry and Community Health Sciences, Institute for Child and Maternal Health, The University of Calgary, Calgary, AB, Canada
Introduction: This chapter presents the analysis of physician-diagnosed International Classification of Diseases (ICD version 9) disorders and diseases associated with autism spectrum disorders (ASD) in a 16-year pediatric cohort.
Materials and Methods: The sample (n = 47,180; 62% male) consisted of children in the Alberta Health Services Calgary Health Region catchment under the age of 3 years, who received any physician-assigned ICD 9 diagnosis before the age of three between April 1993 and December 31, 1994. There were 111 females and 609 males with ASD diagnosed at any time between 1993 and 2010. The results detail the 16-year odds ratio (OR) associations of ASD diagnosis within the major classes of international classification of diseases (ICD 9) stratified by age and sex in the cohort. Further, for those suffering from ASD and any other disorder or disease, the analysis presents by sex, age, and duration, the proportions of all index physician-assigned ICD diagnoses, arising significantly before and after the index ASD diagnosis.
Results: The rate of treated ASD in the cohort was 1 in 65 and the 16-year population rate of ASD was 62 per 10,000. For males with an ASD over the 16 year period, the ORs were significantly greater than the value one for 15 of the 17 main ICD classes and for 10 of the main ICD classes for females. Different age strata presented a more specific account of the main ICD class OR profiles. More specifically, 28 ICD disorders significantly preceded and 95 ICD disorders significantly followed ASD for females. Thirty-eight ICD disorders significantly preceded and 234 ICD disorders significantly followed ASD for males.
Conclusions: The results largely confirm past studies focusing on more constrained sets of ASD morbidity. The age-stratified ORs gauge the order of risk in time for the cohort. The proportions of specific ICD disorders arising before and after ASD may be useful in respect to informing basic ASD research and ASD clinical management. Limitations are discussed.
Introduction
Between one in 65 and one in 88 children born in the United States and Canada are diagnosed with ASD (1–4). To date little detailed knowledge has emerged about the different types of morbidity associated with ASD. Based on a literature review spanning the period between 1991 and 2018, it was apparent that most of the ASD morbidity studies have generally focused only on concurrent or prodromal disorders, primarily psychiatric disorders, occasionally physical disorders, and at best only a few disorders within any given study (5–22). The literature review focused on titles containing the terms autism and the stem “morbid.” The literature illustrated a basic limitation of the morbidity research design to inform an understanding of the etiology of ASD or its clinical management.
Background
While past studies have identified the ambiguity of comorbidity and multimorbidity definitions (23). Others have sought to remedy the situation, by expanding the conceptualization of comorbidity, multimorbidity, concurrent, and associated disorders. Progress has been made in terms of position papers identifying the scope of the definitions required to begin to understand the associations of disorders, both phenomenological and temporal (24, 25). Jakovljević, in a seminal paper, introduces the terms “anosognosia1” in relation to the medical field's approach to the study of multimorbidity, given its range and complexity and the observation that comorbidity and multimorbidity are “under-recognized, under-diagnosed, under-estimated and under-treated” (25). For example, a range of definitions for morbidity including comorbidity and multimorbidity have emerged to better communicate the concepts underpinning the potential relationships of disorders and diseases as these arise in the individual.
In its simplest form, comorbidity exists in from 35 to 88% of all ill people and refers to two or more concurrently co-existing diseases or disorders (25). Researchers have extended the possible definitions of morbidity, comorbidity, and multimorbidity to include terms such as hypercomorbidity and hypocomorbidity that refer to co-occurrence that is greater or lesser than chance, respectively (25). Time necessarily becomes a principle operator in distinguishing sets of definitions related to the directionality of comorbid diseases and their complex nature (26). Also of particular importance is that of each disease's probability in relation to the others' onset (26).
Multimorbidity is defined as the coexistence of multiple chronic diseases or conditions within an individual (27). The definitions of morbidity with inclusion of the often transient nature of temporal morbidity further complicates how morbidity is conceptualized. For example, disorders that are not always present or appear resolved in the individual either before or after the time of ASD onset, may be over-represented in the population of ASD-diagnosed individuals. Definition of morbidity, for the purpose of this paper, required extension to include the concept of temporality and associated transient diseases or disorders, not necessarily present at the time of index ASD diagnosis, yet present at some past or future time in proportions greater than that expected by chance alone. The present study extends previous work on ASD (28) in describing the full range of unique International Classification of Diseases (ICD) diagnoses associated in time with ASD.
Accompanying updated definitions aiding the conceptualization of morbidity is the advantage of access to large databases, such as the one on which present study and similar studies have been based (29–31). The 16-year database has been the source of numerous publications related to identifying key relationships between biomedical, physical, and mental disorders, in addition to the influence of the temporal occurrence of particular classes of disorders (e.g., mental disorders) in relationship to serious biomedical and somatic disorders (28–33). Identifying a cohort under the age of 3 years within a time frame and following the progress of disorders diagnosed within this group over a 16-year period made possible the examination of the relative emergence of temporal morbidity in relation to the index diagnosis of ASD.
Many disorders are transient (e.g., infections), however, once diagnosed, ASD persists as a comorbid diagnosis in time with all subsequent diagnoses.
Study Objective
The proposition under study is that many temporal physical and biomedical morbidities, transient or persistent, will arise in significant proportions within the ASD-diagnosed population in comparison to those without ASD. When over-represented (hypermorbidity), the pattern of morbidity associated with specific diagnoses in addition to main classes, may provide a better understanding of the etiology or the sequelae of ASD. Etiology might inform basic research via the study of specific infections and /or inflammatory processes as well as early diagnosis. Sequelae may facilitate ASD management and care planning when diseases or disorder appear above a given threshold. Of note is that by definition, representation of associated disease might arise in at least one of four main categories in relation to a target (pivot) diagnosis, ASD in this case: significant and non-representative of the sample, non-significant, or significantly before or after an index “pivot” diagnosis (e.g., ASD). Additionally, yet beyond the scope of the present study are the additional categories of transient vs. persistent.
Materials and Methods
The sample from which the sub-sample for this study2 derives and the methods employed in this study have been described in detail (28–31, 34). This study extends previous work in describing the specific disorders arising significantly before or after index ASD, the pivot diagnosis.
For this study, a sample was constructed consisting of a regional cohort under 3 years of age between April 1, 1993 and December 31, 1994 having presented with any physician-diagnosis. All ICD 9 diagnoses assigned to this set of unique individuals over the next approximately 16 years up to November 2010 were merged by unique individual and truncated at maximum age <18 years. Those having an ASD diagnosis were labeled as a group along with all their associated ICD diagnoses, and individuals with unlinked diagnoses making up the comparison group.
The odds ratios (OR) were calculated for this group in relation to the remaining 17 main classes of ICD diseases and disorders (excluding mental disorders). The standard OR formula takes the form of ad/bc, with each of the cells representing a (neither condition), b (one condition), c (the other condition), and d (both conditions) in the 2X2 cross-tabulation. The results were represented in tables and graphs separately for each sex and stratified by age groups. ORs with lower or upper 95% confidence intervals (LCI, UCI) greater or less than the value one were considered to be statistically significant (p < 0.05) with z set at value 1.96. Significance and 95% confidence intervals are noted in each table but not in the graphics. In the graphics, lines for each age strata connect the different main ICD classes for ease of comparison within and between age strata.
For those diagnosed with both ASD and any ICD disorder or disease, a count of the occurrence of each ICD diagnosis arising before and after the pivot ASD diagnosis were calculated within each diagnosis and the proportions arising before and after ASD were compared based on upper and lower 95% confidence intervals defined by the standard formula. Additionally, the average duration in days arising before and after ASD, and the average age in years of each disorder were calculated and tabled. Due to the large number of ICD diagnoses (>900), only those with significant proportions arising before and after ASD were tabled. Where the value zero occurred in a cell, the value one was substituted for the purpose of estimating either ORs or proportions and their 95% CIs. In determining the importance of a particular association both the proportion before or after ASD and the proportion before or after of the total sample need to be taken into account.
Note that in the tables the all-age group OR and cell values were the same as the < 3 age group OR cell values due to the method used to construct the sample. For example, the only individuals that could be linked in subsequent ages >3 years of age were the individuals within the cohort that remained in the catchment and received a physician-assigned diagnosis at any time over the next 16 years. Variations in the other age groups' ORs and cell values represent actual variations for these main classes of ICD disorders within the age groupings of the cohort over time. While attrition is in this sense may be confounded with absence of disease, the presence of a significant and representative OR association is actual.
Mental disorders were not considered in the present analysis in terms of the calculated ORs, as the different algorithm structure required for analysis was beyond the scope and resources of the present study. Including the temporal morbidity of specific mental disorders other than ASD was possible as output due to the logic structure in the algorithm giving rise to the examination of the specific proportions of ICD diagnoses arising before or after ASD (Table 4 in Supplementary Material).
Results
There were 17,898 females in the 1993–1994 cohort sample with an average of 61 physician-diagnoses and a range between 1 and 754 diagnoses over the next 16 years for a total of 1,092,752 diagnoses. There were 111 females with ASD diagnosed at some point over the next 16 years. The female mean age of first ASD diagnosis was 7.5 years (std. dev. 3.6) with range from 0 to 16 years.
There were 29,191 males in the sample with an average of 66 physician-diagnoses and a range between 1 and 1,548 diagnoses for a 16-year total of 1,914,955 diagnoses. There were 609 males with ASD diagnosed at some point over the next 16 years. The male mean age of first ASD diagnosis was 7.8 years (std. dev. 3.2) with range from 0 to 17 years.
Of the total 16-year sample (all ages) one in 65 children in the 1993–94 cohort had an ASD diagnosis. The overall all-age OR comparison for the presence or absence of ASD by major classes of ICD diagnoses for males and females (ranked from highest to lowest by males) are shown in Table 1. For males with an ASD over the 16 year period, the ORs were significantly greater than one for all ICD main classes, with the exception of injury poisoning and complications of pregnancy and childbirth. The ORs for females with an ASD over the 16 year period were significantly greater than the value one for ICD main classes: sense organs, nervous system, circulatory system, congenital anomalies, endocrine, digestive system, skin subcutaneous tissue, neoplasms, genitourinary system, and injury/poisoning. The ORs for males with ASD were substantially higher than females for the ICD major classes: sense organs, respiratory system, and symptoms signs ill-defined conditions. The ORs for females with ASD were slightly higher than males for the ICD major classes: nervous system, circulatory system, congenital anomalies, endocrine, digestive system, skin subcutaneous tissue, neoplasms, genitourinary system, and injury/poisoning,
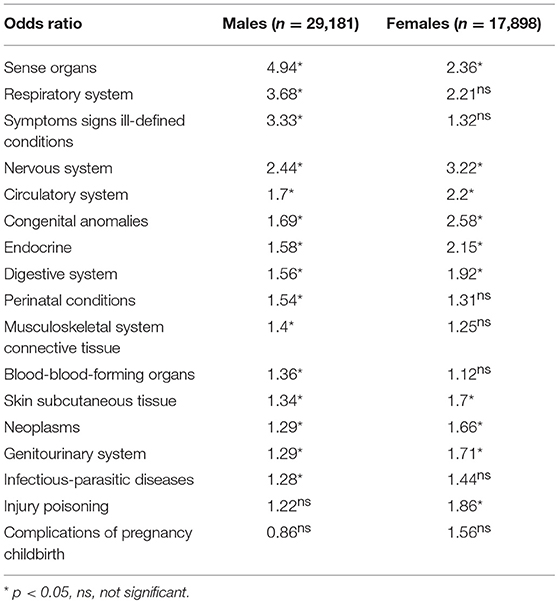
Table 1. The all-age odds ratios comparison for the presence and absence of ASD by major classes of ICD 9 diagnoses ranked from highest to lowest by males.
Figure 1 shows for comparison the ASD OR values from Table 1 by major class ICD 9 diagnosis for males and females (all ages), ranked from highest to lowest by males. For the cohort across all ages, the greatest OR for males given an ASD over 16 year was sensory organs disorders, while for females it was nervous system disorders.
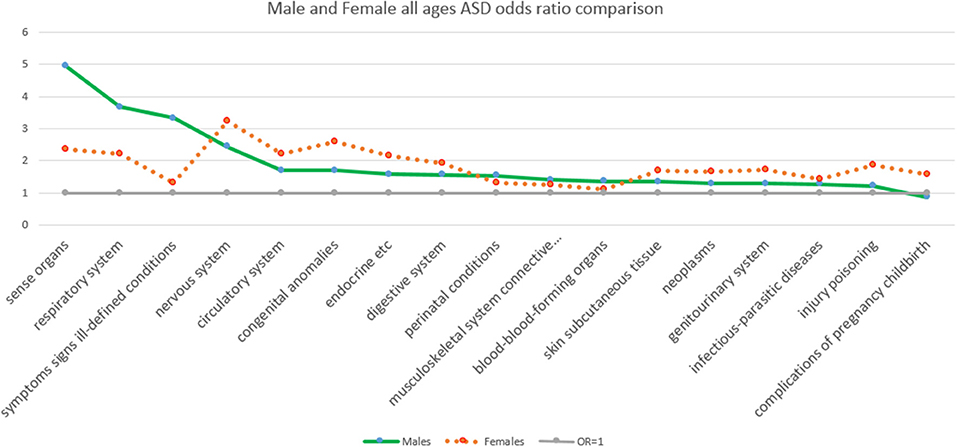
Figure 1. The odds ratios comparison of the presence and absence of ASD by major class ICD 9 diagnosis ranked by sex from highest to lowest by males.
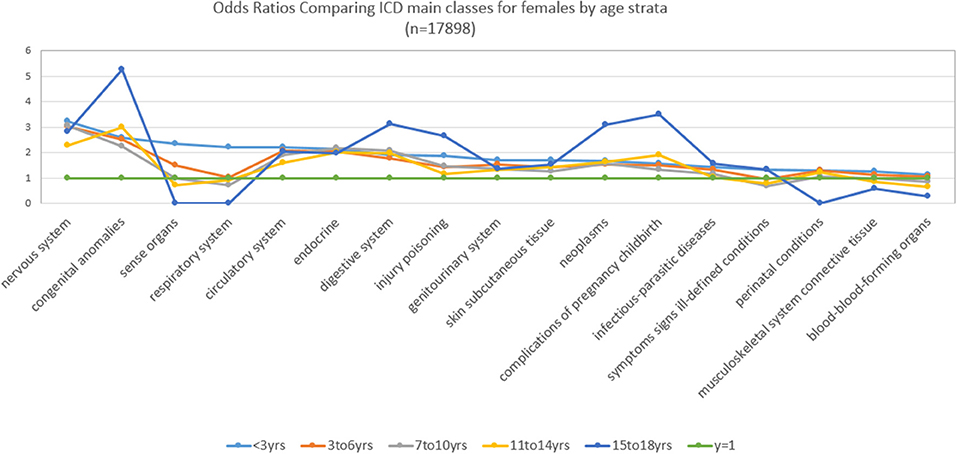
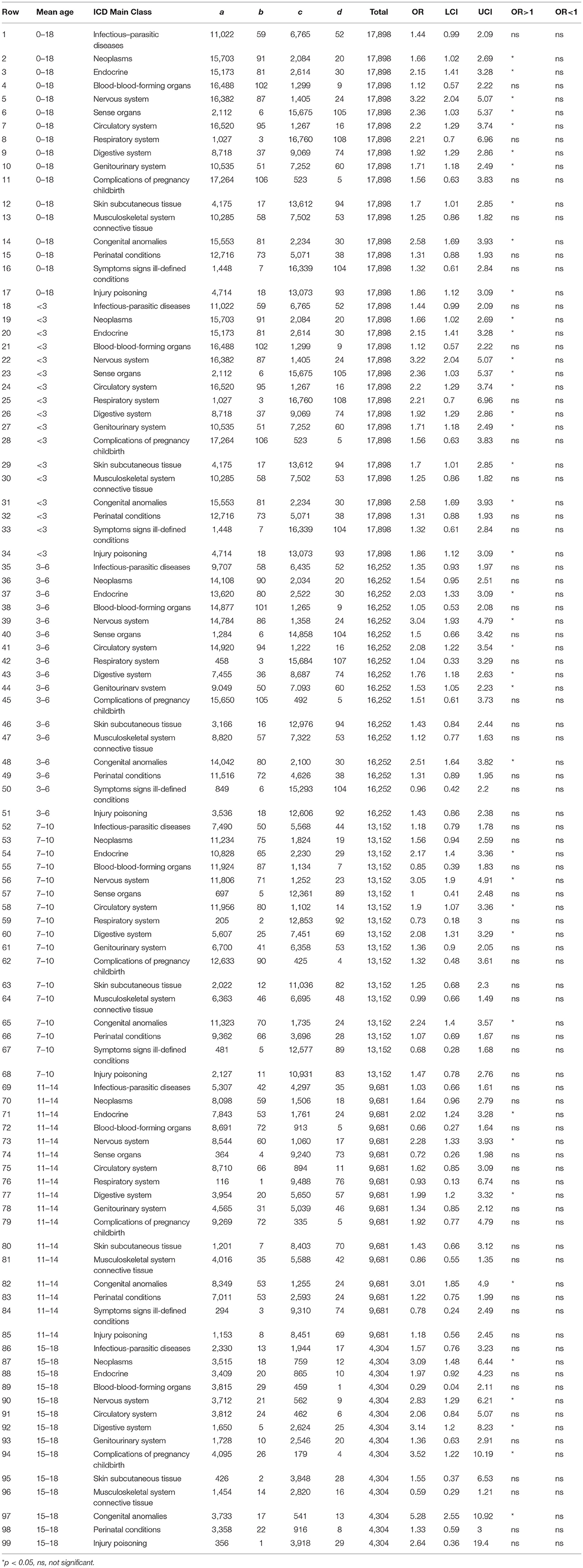
Figure 2 and Table 2 show for females with an ASD over 16 years, the ORs stratified by age groups, which present a somewhat different picture. For females, the ORs (value > 2) that were significantly and representatively greater at the earliest age for each main ICD class follows: 15–18 years, congenital anomalies; 15–18 years, complications of pregnancy childbirth; <3 years, nervous system; 15–18 years, neoplasms; 3–6 years, nervous system; <3 years, congenital anomalies; <3 years, sense organs; <3 years, circulatory system; <3 years, endocrine; 7–1 years, 0, digestive system.
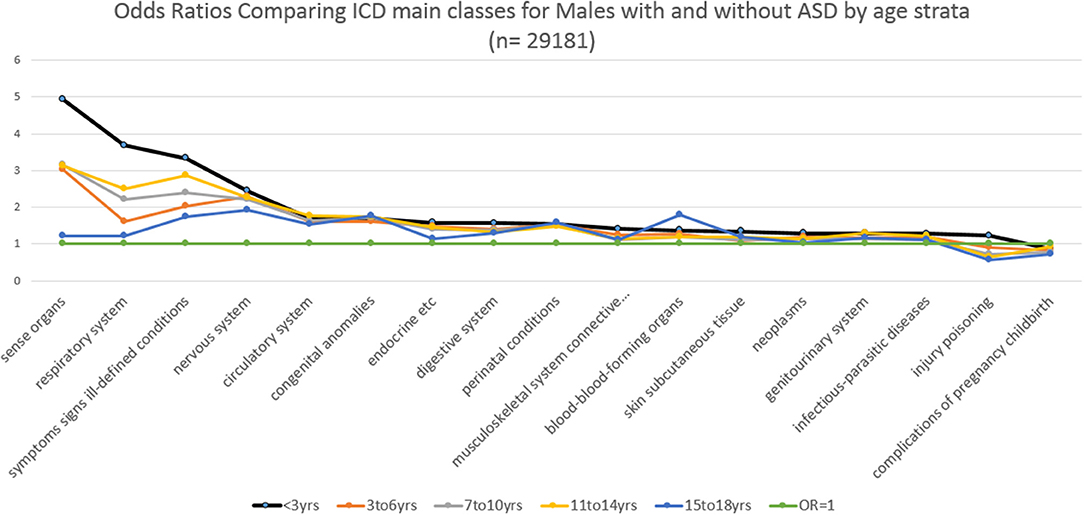
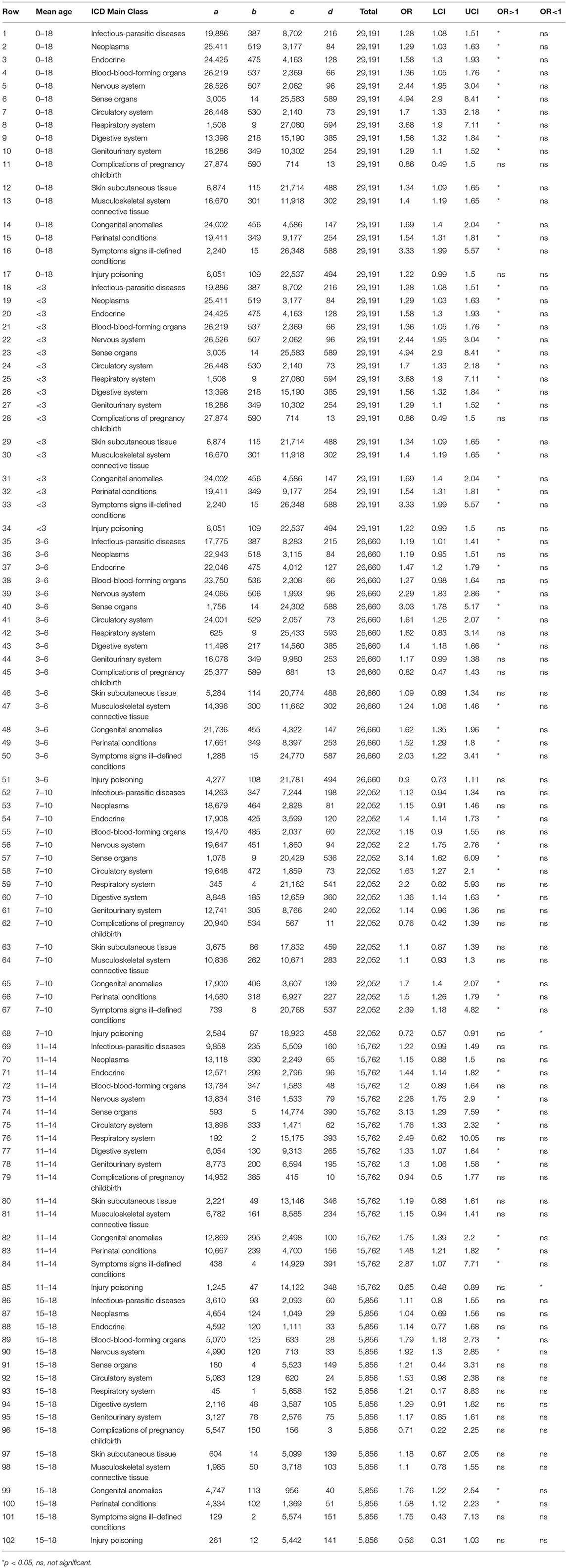
Similarly, Figure 3 and Table 3 show for males with an ASD over 16 years, the ORs stratified by age groups. For males, the ORs (value >2) that were significantly and representatively greater at the earliest age for each main ICD class follow: <3 years, sense organs; < 3 years, respiratory system; < 3 years, symptoms signs ill-defined conditions; 7–10 years, sense organs; 3–6 years, sense organs; < 3 years, nervous system; 7–10 years, symptoms signs ill-defined conditions; 3–6 years, nervous system. Additionally for males, one OR (value < 1) was significantly and representatively less at the earliest age 7–10 years. for injury and poisoning.
Table 4 in Supplementary Material is comprised of 4 sections showing in rows the comparisons of the proportions of distinct physician-assigned ICD diagnoses for males and females arising significantly before and after ASD. The average age in years of individuals within each row is shown together with the average duration in days the ASD arose before or after each ICD diagnosis. Table 4 in Supplementary Material is ordered by row number and the ascending numeric sequence of the ICD code for each disorder, first for females in rows 1–28 (28 disorders) where ASD rose significantly before the corresponding ICD disorder, second for females in rows 31–125 (95 disorders) where ASD rose significantly after the corresponding ICD disorder. For males in rows 126–165 (38 disorders), ASD rose significantly before the corresponding ICD disorder and finally, for males in rows 166–401 (234 disorders), where ASD rose significantly after the corresponding ICD disorder.
Examples of disorders with the highest sample proportions diagnosed after and before ASD follow. For females ASD diagnosis preceded the following disorders with the highest counts ranging from 44 to 7% of the females sample, respectively: psychoses of childhood (299), hyperkinetic syndrome (314), emotional disorder child/adol (313), neurotic disorders (300), conduct disturbance nec (312), depressive disorder nec (311), other viral disease (78), adjustment reaction (309), screen-heart/resp/gu disorder (V81), disorder of menstruation (626), gastritis and duodenitis (535).
For females ASD diagnosis came after the following disorders with the highest counts ranging from 74 to 30% of the females sample, respectively: health supervision child (V20), otitis media, suppur/nos (382), ac up resp inf multiple sites/nos (465), acute nasopharyngitis (460), general symptoms (780), single liveborn (V30), disorders of conjunctiva (372), specific develop delays (315), contact dermatitis (692), ill-defined intest inf (009), nonsuppur otitis media (381), ac bronchitis/bronchiol (466), nutrit/metab/devel symp (783), general medical exam (V70), candidiasis (112), and resp sys/other chest symp (786).
For males ASD diagnosis preceded the following disorders with the highest counts ranging from 47 to 7% of the male sample, respectively: psychoses of childhood (299), hyperkinetic syndrome (314), other viral disease (78), depressive disorder nec (311), other soft tissue disorder (729), screen-heart/resp/gu disorder (V81), and special symptom nec (307).
For males ASD diagnosis came after the following disorders with the highest counts ranging from 79 to 34% of the male sample, respectively: health supervision child (V20), otitis media, suppur/nos (382), ac up resp inf multiple sites/nos (465), acute nasopharyngitis (460), general symptoms (780), ill-defined intest inf (009), disorders of conjunctiva (372), acute bronchitis/bronchiol (466), specific develop delays (315), nonsuppur otitis media (381), acute pharyngitis (462), skin/other integument symp (782), asthma (493), resp sys/other chest symp (786), contact dermatitis (692).
Summary of Results
The results of the analysis of ASD and the main ICD classes of disease and disorder indicated a significant association that varied with age groups. Given the construction of the sample, the age groupings provided a proxy for the temporal progression of the observed class associations, for which some main class ORs were more pronounced at various ages for one or both sexes. The significant OR associations with main ICD classes were less frequent for females.
The analysis results for specific ICD diseases and disorders provided more precise information about the temporal relationship of each disorder and ASD. For example, concurrently examining the mean age, the number of cases, mean duration in days that the specific diagnosis arose either before or after ASD, together with the significance of their proportions provided additional information related to the temporality of etiology and sequelae of ASD morbidity, especially when the whole sample proportions (e.g., >30%) the before or after ASD counts of each diagnosis were additionally examined for males and females.
Discussion
The estimated prevalence of ASD diagnosis in the cohort sample of treated children was one in 65, and as estimated from the previous study based on those under the age of 19 years (28) was 62 per 10,000 in the entire population, a result similar to other United States and Canadian studies (1, 2) and higher than past studies (1, 35). One difference was that prevalence in the present 1993-94 cohort was prospective in that the value was calculated based on an ASD diagnosis made at any time over the next 16 years. An observation about Canadian data is that there have been few datasets that permit a conclusive estimate of ASD prevalence (36). The average age of ASD diagnosis in the present study was older than that previously reported for all of Alberta (36). The different study purposes, foci, and designs may have accounted for observed differences in age of diagnosis. The provincial study focused on the prospective Alberta Perinatal Health Program cohort in which the births were actively monitored over the course of the study (36), while the present study focused on the 16-year main ICD class and specific ICD disorder temporal morbidity associated with ASD in a group under 3-years of age between 1993 and 1995.
The emerging field of ASD comorbidity and multimorbidity is evolving and complex. The changing concept of morbidity must necessarily shift health systems and education to configure around multimorbidity from a focus on individual diseases (37). Multimorbidity is the most common chronic condition experienced in significant proportions by older and younger adults: Health system redesign is required to accommodate the emerging body of research (27). Systematic review indicates that the ability to respond clinically to morbidity, comorbidity, and multimorbidity is in its infancy (38).
A number of studies have focused attention on physical disorder comorbidity (39–44) including population studies (45, 46), putative mechanisms underpinning ASD (47–57), and how cross-comorbidity study identifies disease linkage (58), as well as autism-related genes (59) with the potential to identify cause (60).
For basic researchers Table 4 in Supplementary Material might serve as a reference for comparison to other population studies and inform deeper analysis that might provide more insight into the ASD etiology or sequelae, or both. It is important to take into account when reviewing the proportions of the sample with ASD as the pivot diagnosis, the raw counts also represent a portion of the total female or male sample. For example, in Table 4 (Supplementary Material), row 31, ASD followed 74% of female cases (n = 43 of 51) with ill-defined intestinal infection (009) representing 39% (n = 43 of 111) of the female sample with that particular disorder. In Table 4 (Supplementary Material), row 169, for males ill-defined intestinal infection (009) arose in 85% (n = 335 of 393) representing 55% (335 of 609) of the sample. For both males and females, the average age was 7 years and the average duration between the diagnosis ill-defined intestinal infection (009) and ASD was 1,885 and 1,890 days, respectively. The closeness of the age and average duration before ASD for both males and females is interesting, given the preponderance of males that suffer from ASD. The brain-gut axis is an increasing popular focus of research in recent years (60–62). It is possibly mere coincidence, even though many infectious and allergic diseases in the sample had this same profile, that males and females in a population are diagnosed with ASD about the same period on average after suffering from this gastro-intestinal disorder. Similarly, the present study also identifies that sensory and respiratory disorders occur with substantial magnitude before ASD. In respect to development of biome via maternal inoculation via natural vaginal delivery, study has identified those born under conditions of augmented birth (e.g., cesarean section) are at higher risk of ASD (3). In alignment, the present study indicates that ASD children may have compromised immune systems, possibly associated with the brain-gut axis (61). Comparing the present study findings to other studies focusing on morbidity, there is similar overlap in the association of inflammatory processes, infection, and the emergence of psychiatric disorders thought to have organic origins, such as schizophrenia (63–65).
How might a clinical temporal, transient, hypercomorbidity profile case stack up, against Table 4 in Supplementary Material? The present work may contribute in the following ways. In clinical practice repeated visits of any child above a threshold (possibly the before or after counts of disorders in Table 4 (Supplementary Material)) may signal the need for more detailed investigation, such as thorough assessment of the child's having attained developmental milestones. This seems a sweeping and costly generalization for any practice guide based on a single diagnosis. However, by examining any given child's etiological morbidity profile across multiple diseases and disorders might possibly reveal a more valid constellation of thresholds, the limitations of the present study notwithstanding. In practical terms, the proportions presented in Table 4 (Supplementary Material) serve as points of reference or signposts. For a clinician treating ASD, interpreting the overall importance of Table 4 in Supplementary Material is likely most relevant to the clinician's reference case, in other words, the case the clinician is presently treating.
With the exception of specific developmental delays, almost all psychiatric disorders were diagnosed after ASD ranging from 62 to 98% of the sample for females. Similarly for males, psychiatric disorders followed ASD ranging from 56 to 100% of the sample with index psychiatric diagnoses. Before ASD diagnosis was established, pre-ASD psychiatric morbidity was monotonic, that is only specific developmental delay (315) was diagnosed. Any explanation underpinning these results is speculative. For instance, ASD literacy in the mid-1990s may have been low and not favored in psychiatric curricula or graduate continuing medical education. Given the increase in the annual rate of ASD diagnosis between 1993 and 2010 within the catchment area (28), this explanation is plausible. Furthermore, Child and Adolescent Psychiatry only formed in 2010 as a specialization recognized by the Canadian Royal College of Physicians and Surgeons, which could have influenced the dearth of specialized training and sub-specialization in developmental disorders. Hence, it is likely that psychiatric-training-based recognition of ASD was different in the late 1990's and early 2000's (about 7 years being the average age of ASD diagnosis).
Limitations
The present work has important limitations. One main limitation of the present study is that each major class or individual ICD diagnosis and specific ICD diagnosis proportions before and after ASD was calculated independently of one another. This limitation was addressed in part by stratifying the main classes by age groups and recording the average age and duration before or after ASD, where ASD and the ICD disorder both occurred in the same individual.
Furthermore, it was not possible to examine the linkage of the dataset employed in this study with databases containing prescription data, laboratory results, or to delve further into treatment outcomes via case review, or genetic analysis of biological samples. One contemporary database that links primarily pharmacological prescription data is the United Kingdom's primary care Health Improvement Network (THIN) database (66). A current literature search for the terms “THIN database multimorbidity” produced zero results. A similar search for “THIN database comorbidity” produced 18 results, while “THIN database morbidity” produced 247 results. The majority of these results were focused on a minimal number of associated diseases or disorders and/or prescriptions. Furthermore, evaluation of the THIN database indicated that information loss due to incomplete mapping of medical and drug codes, as well as data structure in the Common Data Model used in THIN limits, its use for all possible epidemiological evaluation studies (66). The database employed in the present study represented the formal physician diagnoses (~90 Million) for the health service seeking population in the Calgary Health Zone (~0.75 Million). As noted in previously published limitations respecting this database, over-diagnosis of common disorders, under-diagnosis of rare disorders and misdiagnosis are all likely represented, albeit minimally, in the data (29, 31). Also, the algorithms applied to the data were developed specifically to examine the temporal associations of the diagnosed diseases and disorders. The approaches taken in analysis with this large dataset are relatively novel, as indicated by the dearth of similar studies, such as those emerging from the THIN database. To date the published studies based on the present database have provided largely broad stroke findings, which, while to some extent useful, are signposts that point to the need for further research and development. The present results extend these findings with a description the temporal order of transient hypermorbidity, not only by the proxy of age for the main ICD classes, but also in relation to the full range of specific ICD diagnoses. The provision of age and duration before and after the pivot ASD add value, possibly bringing information into a framework useful to both research and clinical practice.
The results of population-based analysis of multimorbidity are complicated and not necessarily straight forward or intuitive. The results point to some potential possibilities in terms of mechanism, yet there is no possibility that studies focusing only on associative morbidity are conclusive. As with the studies that illustrate a potential confounding of treatment with outcome diagnoses such as ulcerative colitis or cancer (29, 31), confounding exists between epigenetics, individual differences, diathesis, and, importantly, long-term unstudied adverse effects of treatment. While less likely to be the case with diseases, such as ulcerative colitis, the results of this population-based study, where ASD diagnosis alone is the key variable, must be considered as speculative.
Conclusions
The present work extends the understanding of temporal, transient hyper-multimorbidity associated with ASD. It confirms the ASD association with gastrointestinal problems and immunological disorders, sensory, and neurological disorders, which have been identified in more constrained studies of comorbidity.
The recently published papers from this population dataset have been informative in terms of signaling the importance of considering mental disorders in relation to physical or biomedical disorders (29–31, 34) and, in the cases of ulcerative colitis and cancer, identifying potential mechanism underlining individual vulnerability (diathesis) or the confounding of the physical or biomedical disease with treatment, or both (29, 31). For example, in both cancers and ulcerative colitis, neuroleptics may disrupt immunological cell-cell communication and represent long-term adverse effects associated with mental disease treatment. This hypothesis is speculative, providing only a direction for detailed basic research.
In conclusion, the present study has the advantage of examining multiform morbidity in a population. The types of analyses including ORs by all ages, stratified by age groups and the proportions before and after any pivot ASD resulted in diagnostic profiles representing novel information potentially informing both basic research, clinical education and practice. The relationship between ASD and the main classes of ICD disorder stratified by age gives a very general indication of the importance of broad diagnostic groupings at different ages and illustrates the need to carefully consider the multiple temporal associations of ASD and specific ICD disorders. The greatest amount of information possibly useful for clinicians is in the proportions of disease before and after ASD, particularly when considered by frequency, age and duration.
Next Steps
It is apparent in considering the study of temporal morbidity in population-based data sets, going forward may require a standardized approach in order for study results to be comparable. Further, noting that the ORs and the specific ICD proportions were independent in the calculation of each main ICD class, hence the resulting ORs (Tables 1–3) and proportions (Table 4 in Supplementary Material) were related only by age and duration before or after ASD. The most important next step in the development of analysis algorithms is to advance an approach to the study of multimorbidity that compares a different matrix format representing the temporal order of specific diagnoses in individuals across all specific ICD diagnoses. The highest levels of observed sequences of disorders that arise and lead to a particular disorder or set of disorders, such as ASD, would represent more a precise analysis than that presently presented. A preliminary algorithm that accomplishes this precision is currently undergoing validation testing.
Author Contributions
DC conceptualized the study, organized the data, conducted analyses, and wrote the paper.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2018.00635/full#supplementary-material
Footnotes
1. ^ A deficit of self-awareness, a condition in which a person (in this case system) seems unaware of its existence (e.g., multimorbidity).
2. ^ This study was approved under Ethics ID: REB_15-1057.
References
1. Bertrand J, Mars A, Boyle C, Bove F, Yeargin-Allsopp M, Decoufle P. Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics (2001) 108:1155–61. doi: 10.1542/peds.108.5.1155
2. Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Aut Res. (2012) 5:160–79. doi: 10.1002/aur.239
3. Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML. Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990-1998) and Education Research (1997-2007) databases. JAMA Pediatr. (2013) 167:959–66. doi: 10.1001/jamapediatrics.2013.2904
4. Diallo FB, Fombonne É, Kisely S, Rochette L, Vasiliadis HM, Vanasse A, et al. Prevalence and correlates of autism spectrum disorders in quebec: prevalence et correlats des troubles du spectre de lautisme au Quebec. Can J Psychiatry (2018) 63:231–9. doi: 10.1177/0706743717737031
5. Mattila ML, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M, et al. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: a community- and clinic-based study. J Aut Dev Dis. (2010) 40:1080–93. doi: 10.1007/s10803-010-0958-2
6. Zachor D, Yang JW, Itzchak EB, Furniss F, Pegg E, Matson JL, et al. Cross-cultural differences in comorbid symptoms of children with autism spectrum disorders: an international examination between Israel, South Korea, the United Kingdom and the United States of America. Dev Neurorehabil. (2011)14:215–20. doi: 10.3109/17518423.2011.568468
7. Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS ONE (2012) 7:e33224. doi: 10.1371/journal.pone.0033224
8. Strunz S, Dziobek I, Roepke S [Comorbid psychiatric disorders and differential diagnosis of patients with autism spectrum disorder without intellectual disability]. Psychother Psychosom med Psychol. (2014) 64:206–13. doi: 10.1055/s-0033-1358708
9. Larson FV, Lai MC, Wagner AP, MRC AIMS Consortium, Baron-Cohen S, Holland AJ. Testing the “Extreme Female Brain” theory of psychosis in adults with autism spectrum disorder with or without co-morbid psychosis. PLoS ONE (2015) 10:e0128102. doi: 10.1371/journal.pone.0128102
10. Peterson BS, Goh S, Dong Z, Dager SR, Corrigan NM, Shaw DW. Brain lactate as a potential biomarker for comorbid anxiety disorder in autism spectrum disorder-reply. JAMA Psychiatry (2015) 190–1. doi: 10.1001/jamapsychiatry.2014.2425
11. Chiang H.-L, Gau S. S.-F. Comorbid psychiatric conditions as mediators to predict later social adjustment in youths with autism spectrum disorder. J Child psychol Psychiatry 57:103–11. doi: 10.1111/jcpp.12450
12. Pan P.-Y, Yeh C.-B. The comorbidity of disruptive mood dysregulation disorder in autism spectrum disorder. Psychiatry Res. (2016) 108–9. doi: 10.1016/j.psychres.2016.05.001
13. Frye RE, Rossignol DA. Identification and treatment of pathophysiological comorbidities of autism spectrum disorder to achieve optimal outcomes. Clinical medicine insights. Pediatrics (2016) 10:43–56. doi: 10.4137/CMPed.S38337
14. Koski S, Gabriels RL, Beresford C. Interventions for paediatric surgery patients with comorbid autism spectrum disorder: a systematic literature review. Arch Dis Childhood (2016) 101:1090–4. doi: 10.1136/archdischild-2016-310814
15. Brookman-Frazee L, Stadnick N, Chlebowski C, Baker-Ericzén M, Ganger W. Characterizing psychiatric comorbidity in children with autism spectrum disorder receiving publicly funded mental health services. Autism (2017) 22:938–52. doi: 10.1177/1362361317712650
16. Sokolova E, Oerlemans AM, Rommelse NN, Groot P, Hartman CA, Glennon JC, et al. A causal and mediation analysis of the comorbidity between attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). J Aut Dev Disor. (2017) 47:1595–604. doi: 10.1007/s10803-017-3083-7
17. Stadnick N, Chlebowski C, Baker-Ericzén M, Dyson M, Garland A, Brookman-Frazee L. Psychiatric comorbidity in autism spectrum disorder: correspondence between mental health clinician report and structured parent interview. Autism (2017) 21:841–51. doi: 10.1177/1362361316654083
18. Espluga-Frigola N, Cardoner N, Pàmias-Massana M, Palao-Vidal DJ. Comorbidity of autism spectrum disorder and bipolar disorder. Actas espanolas de psiquiatria. (2017) 79–88.
19. Flor J, Bellando J, Lopez M, Shui A. Developmental functioning and medical Co-morbidity profile of children with complex and essential autism. Aut Res. (2017) 10:1344–52. doi: 10.1002/aur.1779
20. Haruvi-Lamdan N, Horesh D, Golan O. PTSD and autism spectrum disorder: co-morbidity, gaps in research, and potential shared mechanisms. Psychol Trauma 10:290–9. doi: 10.1037/tra0000298
21. Bozzi Y, Provenzano G, Casarosa S. Neurobiological bases of autism-epilepsy comorbidity: a focus on excitation/inhibition imbalance. Eur J Neurosci. (2018) 47:534–48. doi: 10.1111/ejn.13595
22. Kantzer AK, Fernell E, Westerlund J, Hagberg B, Gillberg C, Miniscalco C. Young children who screen positive for autism: Stability, change and “comorbidity” over two years. Res Dev Disabil. 72:297–307. doi: 10.1016/j.ridd.2016.10.004
23. van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity, Eur J General Pract. (1996) 2:65–70. doi: 10.3109/13814789609162146
25. Jakovljevic M, Ostojic L. Comorbidity and multimorbidity in medicine today: challenges and opportunities for bringing separated branches of medicine closer to each other. Psychiatria Danubina (2013) 25(Suppl. 1):18–28.
26. Bonavita V, De Simone R. Towards a definition of comorbidity in the light of clinical complexity. Neurol Sci. (2008) 29(Suppl. 1):S99–102. doi: 10.1007/s10072-008-0898-1
27. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition – Multimorbidity. JAMA (2012) 307:2493–4. doi: 10.1001/jama.2012.5265
28. Cawthorpe D. Comprehensive description of comorbidity for autism spectrum disorder in a general population. Permanente J (2017) 21:16–088. doi: 10.7812/TPP/16-088
29. Cawthorpe D, Davidson M. Temporal comorbidity of mental disorder and ulcerative colitis. Permanente J. (2015) 19:52–57. doi: 10.7812/TPP/14-120
30. Chartier G, Cawthorpe D. From “Big 4” to “Big 5”: a review and epidemiological study on the relationship between psychiatric disorders and World Health Organization preventable diseases. Curr Opin Psychiatry (2016) 29:316–21. doi: 10.1097/YCO.0000000000000270
31. Cawthorpe D, Kerba M, Narendran A, Ghuttora H, Chartier G, Sartorius N. Temporal order of cancers and mental disorders in an adult population. BJPsych open (2018b) 4:95–105. doi: 10.1192/bjo.2018.5
32. Cawthorpe D, Wilkes TC, Guyn L, Li B, Lu M. Association of mental health with health care use and cost: a population study. Can J Psychiatry (2011) 56:490–4. doi: 10.1177/070674371105600807
33. Wilkes TC, Guyn L, Li B, Lu M, Cawthorpe D. Association of child and adolescent psychiatric disorders with somatic or biomedical diagnoses: do population-based utilization study results support the adverse childhood experiences study? Permanente J (2012) 16:23–6.
34. Cawthorpe D. A novel population-based health index for mental disorder. Permanente J. (2013) 17:50–4. doi: 10.7812/TPP/12-081
35. Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA (2003) 289:49–55.
36. Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 30:125–34. doi: 10.1186/1471-2288-11-2
37. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet (2012) 380:37–43. doi: 10.1016/S0140-6736(12)60240-2
38. Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. (2012) 4:CD006560. doi: 10.1002/14651858.CD006560.pub2
39. Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics (2010) 7:320–7. doi: 10.1016/j.nurt.2010.06.001
40. Ahmedani BK, Hock RM. Health care access and treatment for children with co-morbid autism and psychiatric conditions. Soc Psychiatry Psychiatric Epidemiol. (2012) 47:1807–14. doi: 10.1007/s00127-012-0482-0
41. Inga Jácome MC, Morales Chacòn LM, Vera Cuesta H, Maragoto Rizo C, Whilby Santiesteban M, Ramos Hernandez L, et al. Peripheral inflammatory markers contributing to comorbidities in autism. Behav Sci. (2016) 6:29. doi: 10.3390/bs6040029
42. Somekh J, Peleg M, Eran A, Koren I, Feiglin A, Demishtein A, et al. A model-driven methodology for exploring complex disease comorbidities applied to autism spectrum disorder and inflammatory bowel disease. J Biomed Inform. (2016) 63:366–78. doi: 10.1016/j.jbi.2016.08.008
43. Muskens JB, Velders FP, Staal WG. Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: a systematic review. Eur Child Adolesc Psychiatry (2017) 26:1093–103. doi: 10.1007/s00787-017-1020-0
44. Vohra R, Madhavan S, Sambamoorthi U. Comorbidity prevalence, healthcare utilization, and expenditures of Medicaid enrolled adults with autism spectrum disorders. Autism(2017) 21:995–1009. doi: 10.1177/1362361316665222
45. Garg S, Lehtonen A, Huson SM, Emsley R, Trump D, Evans DG, et al. Autism and other psychiatric comorbidity in neurofibromatosis type 1: evidence from a population-based study. Dev Med Child Neurol. (2013) 55:139–145. doi: 10.1111/dmcn.12043
46. Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics (2014) 133:e54–63. doi: 10.1542/peds.2013-0819
47. Hogart A, Wu D, LaSalle JM, Schanen NC. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol Dis (2010) 38:181–91. doi: 10.1016/j.nbd.2008.08.011
48. Noterdaeme MA, Hutzelmeyer-Nickels A. [Comorbidity in autism spectrum disorders - II. Genetic syndromes and neurological problems]. Zeitschrift fur Kinder- und Jugendpsychiatrie und Psychotherapie (2010) 38:267–72. doi: 10.1024/1422-4917/a000046
49. Bejerot S, Humble MB, Gardner A. Endocrine disruptors, the increase of autism spectrum disorder and its comorbidity with gender identity disorder–a hypothetical association. Int J Androl. 34:e350. doi: 10.1111/j.1365-2605.2011.01149.x
50. Ragunath P, Chitra R, Mohammad S, Abhinand P. A systems biological study on the comorbidity of autism spectrum disorders and bipolar disorder. Bioinformation (2011) 7:102–6.
51. Abbeduto L, McDuffie A, Thurman AJ. The fragile X syndrome-autism comorbidity: what do we really know? Front Genet. (2014) 5:355. doi: 10.3389/fgene.2014.00355
52. Hollocks MJ, Howlin P, Papadopoulos AS, Khondoker M, Simonoff E. Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology (2014) 46:32–45. doi: 10.1016/j.psyneuen.2014.04.004
53. Polyak A, Kubina RM, Girirajan S. Comorbidity of intellectual disability confounds ascertainment of autism: implications for genetic diagnosis. Am J Med Genet B Neuropsychiatr Genet. (2015) 168:600–608. doi: 10.1002/ajmg.b.32338
54. David MM, Enard D, Ozturk A, Daniels J, Jung JY, Diaz-Beltran L, et al. Comorbid analysis of genes associated with autism spectrum disorders reveals differential evolutionary constraints. PLoS ONE (2016) 11:e0157937. doi: 10.1371/journal.pone.0157937
55. Markkanen E, Meyer U, Dianov GL. DNA damage and repair in schizophrenia and autism: implications for cancer comorbidity and beyond. Int J Mol Sci. (2016) 17:E856. doi: 10.3390/ijms17060856
56. Jyonouchi H, Geng L, Streck DL, Dermody JJ, Toruner GA. MicroRNA expression changes in association with changes in interleukin-1ss/interleukin10 ratios produced by monocytes in autism spectrum disorders: their association with neuropsychiatric symptoms and comorbid conditions (observational study). J Neuroinflamm. (2017) 14:229. doi: 10.1186/s12974-017-1003-6
57. Larson FV, Arrand JR, Tantam D, Jones PB, Holland AJ. Copy number variants in people with autism spectrum disorders and co-morbid psychosis. Eur J Med Genet. (2018) 61:230–4. doi: 10.1016/j.ejmg.2017.12.005
58. Rose DR, Yang H, Serena G, Sturgeon C, Ma B, Careaga M, et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav Immun (2018) 70:354–68. doi: 10.1016/j.bbi.2018.03.025
59. Diaz-Beltran L, Esteban FJ, Varma M, Ortuzk A, David M, Wall DP. Cross-disorder comparative analysis of comorbid conditions reveals novel autism candidate genes. BMC Genom. (2017) 18:315. doi: 10.1186/s12864-017-3667-9
60. Mayer EA, Padua D, Tillisch K. Altered brain-gut axis in autism: comorbidity or causative mechanisms? BioEssays (2014) 36:933–9. doi: 10.1002/bies.201400075
61. Vela G, Stark P, Socha M, Sauer AK, Grabrucker AM. Zinc in gut-brain interaction in autism and neurological disorders. Neural Plasticity (2015) 2015:972791. doi: 10.1155/2015/972791
62. Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. (2016) 22:361–8. doi: 10.3748/wjg.v22.i1.361
63. Stevens JR, Langloss JM, Albrecht P, Yolken R, Wang YN. A search for cytomegalovirus and herpes viral antigen in brains of schizophrenic patients. Arch Gener Psychiatry (1984) 41:795–801.
64. Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. Physical illness and schizophrenia: a review of the literature. Acta psychiatrica Scand. (2007) 116:317–33. doi: 10.1111/j.1600-0447.2007.01095.x
65. Prusty BK, Gulve N, Govind S, Krueger GRF, Feichtinger J, Larcombe L, et al. Active HHV-6 infection of cerebellar purkinje cells in mood disorders, Front Microbiol. (2018) 9:1955. doi: 10.3389/fmicb.2018.01955
Keywords: autism spectrum disorder, temporal hypermorbidity, population cohort, ICD-9, ASD-associated morbidity
Citation: Cawthorpe D (2018) A 16-Year Cohort Analysis of Autism Spectrum Disorder-Associated Morbidity in a Pediatric Population. Front. Psychiatry 9:635. doi: 10.3389/fpsyt.2018.00635
Received: 14 June 2018; Accepted: 08 November 2018;
Published: 29 November 2018.
Edited by:
Manuel Fernando Casanova, School of Medicine Greenville, University of South Carolina, United StatesReviewed by:
Vivek Agarwal, King George's Medical University, IndiaHiroshi Kadotani, Shiga University of Medical Science, Japan
Thomas Lefèvre, Université Paris 13, France
Copyright © 2018 Cawthorpe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Cawthorpe, cawthord@ucalgary.ca
 David Cawthorpe
David Cawthorpe