Our Recommendations
- Tropical Medicine Point-of-Care Testing. Update 2022 (2022)
- Manual de Biossegurança Laboratorial (2021)
- Medical device donations: considerations for solicitation and provision (2011)
- WHO Guidelines on Good Manufacturing Practices (GMP) for Herbal Medicine (2007)
- Management of Waste from Injection Activities at District Level (2006)
New Publications
- Guideline on haemoglobin cutoffs to define anaemia in individuals and populations (2024)
- Infection prevention and control measures when caring for patients with suspected or confirmed respiratory diphtheria: a summary (2024)
- Tiene cerdos?-Tienen gusanos? (2024)
- Got Pigs- Got Worms? (2024)
- Laboratory testing for diphtheria in outbreak settings: Interim guidance, 26 January 2024 (2024)
Filter
232
Featured
Recommendations
51
New Publications
90
Language
Document type
No document type
87
Guidelines
65
Manuals
35
Studies & Reports
18
Training Material
12
Fact sheets
5
Resource Platforms
4
Strategic & Response Plan
3
Online Courses
2
Videos
1
Countries / Regions
Latin America and the Carribbean
14
Global
11
Africa
10
Mozambique
6
Brazil
6
Angola
6
Sierra Leone
5
Liberia
5
Nigeria
4
Ghana
3
East and Southern Africa
3
Kenya
2
West and Central Africa
2
Ethiopia
2
India
2
Nepal
1
China
1
Syria
1
Malawi
1
Asia
1
Tanzania
1
Germany
1
Middle East and North Africa
1
Western Pacific Region
1
Bangladesh
1
Sudan
1
Mali
1
South Sudan
1
Somalia
1
Authors & Publishers
Publication Years
Category
General Guidelines
44
Parasitology
42
Medical Devices & Equipment
33
Virology
29
Bacteriology
28
Laboratory Quality Assurance
20
Pharmacy & Technologies
20
Hematology
12
Disease Prevention & Control
12
Laboratory Waste
11
COVID-19
8
Infection Control & Prevention (IPC)
7
Drugs & Medical Equipment
6
Waste Management
6
Pharmacy & Technology
5
Capacity Building
5
Quality Control & Assurance
5
Leishmaniasis
5
Laboratory Technologies
5
Medicines Donations
4
Emergency Health Kits
4
Yersinia pestis (Plague)
4
Neonatal Care
4
Malaria
4
Radiology and Diagnostic
4
Chagas
3
Paediatrics
2
Hepatitis
2
Buruli Ulcer
2
Diarrhoeal Disease/Cholera
2
Guidelines
2
Gynaecology & Obstetrics
2
Maternal Health
2
HIV & STI
2
Key Resources
2
Reproductive Health
2
TB
2
Communicable Diseases
1
Digital Health
1
Family Planning
1
Child Health
1
Disaster Preparedness
1
Schistosomiasis
1
Antimicrobial Resistance
1
Cancer
1
Vector Control
1
Monitoring & Evaluation
1
Pharmacy
1
Cancer Treatment & Resources
1
MPOX - Monkeypox
1
Videos
1
Pediatrics
1
Vaccine-preventable Diseases
1
Health System Strengthening
1
Cardiovascular Diseases
1
Clinical Guidelines
1
Yellow Fever
1
Studies & Reports
1
Other
1
WASH
1
Immunization
1
General Guidelines
1
Health Systems
1
Neurology & Psychology
1
Toolboxes
Diagnostics & Laboratory Guidance
19
Quality Assurance
15
Laboratory Guidance
14
Waste Managment
12
Detection & Diagnosis
12
Laboratory & Sampling
9
Public Health
8
Country specific documents
7
General
6
Genomic sequencing
5
Essential Medicines
5
Biosafety
4
IPC
4
Prevention & Control
4
Medicine Donations
4
Leishmaniasis
4
Clinical Aspects & Diagnostics
4
Plague Prevention & Control
4
WASH
3
Hospital Readiness/Essential Health Services
3
Waste Management
3
Pharmacy
3
Essential Medicines & Devices Lists
3
Falsified & Substandard
3
Pharmacy Practice
3
Supply Chain & Store Management
3
Chagas
3
Studies & Reports
3
Clinical Guidelines
3
Rapid Response
2
Clinical Aspects, Treatment & Prevention
2
Drugs / Medicines / Pharmacy
2
Laboratory
2
Hand Hygiene
2
Training, others
2
Scientific & Research Studies
2
Prices and Patents
2
Pharmacy Management
2
Resuscitation
2
Agriculture incl. animal husbandry
2
General Guidelines for Rational Use
2
MPOX - Monkeypox
2
Overview, Studies & Reports
2
Zika
2
Human Rights & the Right to Health
2
Diarrhoea
2
Infection Control
2
Laboratory & Diagnostics
2
Treatment Guidelines
1
Policies & Strategies
1
HIV testing & services
1
Surveillance
1
Yellow Fever
1
Yellow Fever Prevention & Control
1
Adults
1
Policies & Programmes
1
Plague
1
HIV
1
General Studies & Reports
1
Cholera Prevention & Control
1
SOPs
1
Cholera
1
Reports
1
Laboratory
1
Clinical Guidelines
1
Infection Prevention & Hygiene
1
Clinical Aspects
1
Public Health
1
Infection Control General
1
Essential Medicines & Medical Devices
1
Immunization Guidelines
1
Logistics & Supply
1
Standard Operating Procedure (SOP)
1
Sanitation
1
Pharmaceutical Assessments
1
Rational use of Medicine
1
Traditional & Herbal Medicines
1
General documents One Health
1
Pharmaceutical Dosage Forms
1
Pharmacovigilance
1
Resource Platforms
1
Mental Disorders
1
Capacity Building
1
Guidelines & Manuals
1
Antimicrobial Resistance (AMR)
1
Emergency health kits
1
Emergency health kits
1
Strategies & Roadmaps
1
Clinical Aspects & Diagnostics
1
Studies & Reports
1
Vector control
1
Access to treatment and laboratory
1
Global Overview
1
Drug resistant TB
1
Assessment & Response
1
Education of students & professionals
1
Clinical Guidelines
1
AmR (Region of Americas)
1
WASH
1
Antiretroviral Treatment ART
1
Laboratory
1
Public Health
1
This manual describes some of the strategic, managerial, financial, technical and scientific aspects to be considered in establishing a national EQA programme for clinical laboratories and other testing services at all health care levels
The purpose of this document is to provide interim guidance to laboratories and stakeholders involved in laboratory testing for Middle East respiratory syndrome coronavirus (MERS-CoV).
Training laboratory managers, senior biologists, and technologists in quality management systems is a step towards obtaining international recognition; it is a step that all countries should take. This training toolkit is intended to provide comprehensive materials that will allow for designing and ...
Following review of the latest evidence, WHO recommends that TB-LAMP can be used as a replacement for microscopy for the diagnosis of pulmonary TB in adults with signs and symptoms of TB. It can also be considered as a follow-on test to microscopy in adults with signs and symptoms of pulmonary TB, e...
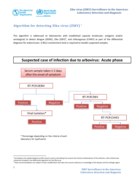
This algorithm is addressed to laboratories
with established capacity(molecular, antigenic and/orserological) to detect dengue (DENV), Zika (ZIKV), and chikungunya(CHIKV) as part of the differential diagnosis for arborviruses. A BSL2 containment level is required to handle suspected samples.
Este algoritmo está dirigido a aquellos laboratorios que cuentan con capacidad instalada para la detección (molecular, antigénica y/o serológica) de dengue (DENV), Zika (ZIKV) y chikungunya(CHIKV),y como parte del diagnóstico diferencial para Arbovirus. Para la manipulación de muestras sosp...
This manual is designed primarily to assist managers of national malaria programmes and national reference laboratory responsible for quality assurance of malaria microscopy control. The information is also applicable to non-governmental organizations and funding agencies investing in quality manage...
July 2016
External quality assessment (EQA) is an important component of quality systems for blood transfusion services. Establishing external quality assessment programmes for screening of donated blood for transfusion-transmissible infections (TTI): implementation guide aims to support WHO member States in ...
Polymerase Chain Reaction (PCR) has significantly helped in early diagnosis and commencement of specific interventions for diseases control. It also plays a critical role in understanding the disease epidemiology and unraveling the transmission dynamics of the disease. This manual intends to p...
L’évaluation externe de la qualité (EEQ) est une composante importante des systèmes qualité des services de transfusion sanguine. L’EEQ est l’évaluation externe de la qualité générale des résultats obtenus par un laboratoire dans l’analyse d’échantillons de contrôle dont le cont...
Every day, fake medicines and medical products are sold at street corners, in open air markets or on unregulated websites in several countries in the African Region. These poor quality, unsafe medicines and pharmaceutical products promote drug resistance and lead to loss of confidence in health prof...
Submitted to the US Agency for International Development by the Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program. Arlington, VA: Management Sciences for Health. Submitted to the United Nations Children’s Fund by JSI, Arlington, VA: JSI Research & Training Institute, Inc....
This report provides an update on the key facets of HIV treatment access, including the latest HIV treatment guidelines from World Health Organization (WHO), an overview on pricing for first-line, second-line, and salvage regimens, and a summary of the opportunities for – and threats to – expand...